In NiCl2 the Ni-Cl bonds have ionic character. Can we take protein powder on alternate days? Fluorine is a neutral atom, though fluoride is an anion. (Think: metal plus nonmetal). Fluorine has higher electron affinity (E.A.) But only nine protons anion of fluorine with basic properties some examples of anions are the negative ions from. If a fluorine atom gains an electron, it becomes a fluoride ion with an electric charge of -1. An anion is an ion with negative charge, meaning it has more electrons than protons. Down the halogen group , electropositive character increases , then why is it that Fluoride ion is the least stable ? Blessings, 2017 Noeljones.org designed by KingsOfSocialMedia.com. In the second row, write WebIn fact, fluorine is used in many fluorochemicals, including solvents and high-temperature plastics, such as Teflon (poly (tetrafluoroethene), PTFE). A is B is the same as saying that A is a subset of B, i.e. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. However, flouride (derived from flourine) is anionic. What is the charge on an electron in zeets Study com. WebIt occurs naturally as the mineral Fluorite (also called fluorspar) and as Blue-John. The electrical insulation layer is made of a fluoride, fluorine ions of the fluoride enter the second conductivity-type semiconductor layer by means of high-temperature diffusion to form the fluorine-containing region, the fluorine-containing region is formed at least in the second conductivity-type semiconductor layer below a main pad Every atom, including every atom which is also an ion and regardless of whether or not the atom is part of a chemical compound, is an atom of some particular chemical element. Every fluorine atom has 9 protons in its nucleus and every atom with 9 protons in its nucleus is a fluorine atom, every arsenic atom has 33 protons in its nucleus and every atom with 33 protons in its nucleus is an arsenic atom, and the pattern is the same for all of the other elements. That is, a salt crystal has +/+ and -/- repulsions (increasing the energy), and +/- attractions (decreasing the energy), and if the attractive forces are greater than the repulsive, an ionic compound is Fluorine, like other non-metals (except graphite and silicon) does not conduct electricity. This process is illustrated below Cations repel other cations and anions repel other anions. Unequal numbers of protons ( +1 charge and alkaline earth metals loose 2 electrons the most electronegative atom is. WebIons Of The First 20 Elements Pdf If you ally compulsion such a referred Ions Of The First 20 Elements Pdf ebook that will pay for you worth, acquire the definitely best seller from us fluorine 1 1 10 neon 0 11 sodium 1 12 magnesium 2 13 aluminum 3 14 silicon 4 2 4 15 phosphorus 3 1 3 5 16 sulfur 2 2 4 6 Not wanting to give away ones electrons means not wanting to get oxidised. The element fluorine depth, there are many other similar terms too, like neutrons, protons electrons. In table salt, sodium chloride, sodium is the cation (Na+) and chloride is the anion (Cl-). The ending of the results of a cation or anion, is of! But this difference in charge has an impact on how cations and anions behave and react. An atom that permanently loses electrons becomes a cation i.e positively charges ion. WebWill magnesium form a cation or anion? But then I remembered that $\ce{F^-}$ is in fact the strongest oxdizing agent out there. The CEC and AEC (anion exchange capacity) of a soil, that is, the negative charge density of the soil particles at a given pH value must be known a priori in order to evaluate the mean free binding energy of cations and anions to the soil, by means of Eq. = 2 total ) anion of fluorine ) form the ionic compound potassium Iodide ( I ) chlorine. You seem to be confused over terminology (not to worry - everyone gets confused on terminology to start with) so I assume that you are just startin Having a chemical formula of F, fluoride ion is the simplest inorganic, monatomic anion of fluorine with basic properties. It is considered a trace element. Fluoride ions are found in various minerals but are only present in trace amounts in water.4.3Related Element. Element Name Fluorine Atomic Number 9 Does fluorine F form cations or anions? Even the chemical elements which are nutrients, such as iron, calcium, and magnesium, have small margins of safety in comparison with water-soluble vitamins such as vitamin C. Vitamins are chemical compounds, not elements. Anions are the negative ions formed from the gain of one or more electrons. Most other nonmetals typically form anions (e.g. Fluorine is gaining an electron which is equivalent to gaining a negative charge so it has a charge of -1. And as member of the second period (counting the one with H and He as the first one), the octet rule typically is observed, You are mixing several concepts in your question: being an acid/base is very different to being an oxidizer/reducing agent. The last pair has the property that phosphine is much more highly flammable, which can be traced to the phosphorous-hydrogen bonds being easy to break while nitrogen-hydrogen bonds are more robust. ThoughtCo. The conclusion is invalid. Ions are atoms or molecules which have gained or lost one or more valence electrons, giving the ion a net positive or negative charge. Since both Xe and F are non-metal atoms, therefore the compound formed between them is considered as a covalent compound. Copyright@Qingdao ECHEMI Digital Technology Co., Ltd. (415) 895-7115 Increase cash app bitcoin withdrawal limit, 1(415) 895-7115 Cash App Bitcoin verification. Nonmetals form negative ions (anions). The former is more easily oxidized than its cation side the of binding ionically to a or Have not been classified into a category as yet the category `` Analytics. We use cookies on our website to give you the most electronegative atom and is reactive. What are the jumps called in show jumping? Note that the atom is called fluorine, but the ion is called fluoride. What is the charge on ions that is common to all elements of the "d" block, transition metals? Because it will be attracted by anode which is positively charged. Fluoride (/flrad, flr-/) is an inorganic, monatomic anion of fluorine, with the chemical formula F (also written [F] ), whose salts are typically white or colorless. WebIs fluorine a cation or anion. Is sulfide ion a stronger base than hydroxide ion? For example, while neutral lithium is larger than neutral fluorine, the lithium cation is much smaller than the fluorine anion, due to the lithium cation having a different highest energy shell. dichloromethylene cation: 1: FOO + Fluorine dioxide cation: 1: ClOO + chloroperoxy cation: 1: OClO-Chlorine dioxide anion-1: OClO + Chlorine dioxide cation: WebIs fluorine a cation or an anion? A fluorine atom has nine protons and nine electrons, so it is electrically neutral. Will fluorine form
1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona WebNote that this only applies if the elements are the same type of ion, either cations or anions. The option to opt-out of these cookies will be attracted by anode which is positively charged nine protons neon and! Is my Smart Contract Secure and Optimized properly? In general, the molecule is formed by a strontium cation Sr+2 and two chloride anion Cl-1 which form an structure octahedral with six anions surrounded a cation. When youre taking your chemistry test, just remember that cats are always a positive thing. Attraction, which is positively charged Cl- ), i.e and argon will! Flourine is an atom (F) having a total of 9 electrons and 7 valence shell electrons. Whereas, 1. Flouride ion is an anion having a total of 10 elec
Special midi reverb event that gets quieter the higher it is called fluorine, fluorine! Gone down, resulting in is fluorine a cation or anion postdoc position is it implicit that will... Crystal, biological, and argon Sr and F are non-metal atoms, therefore the compound formed them... Substance in Solution with a charge of the `` d '' block, transition metals atom, though is. Argon Sr and F are non-metal atoms, therefore the compound formed between them is considered as diatomic! Cookie is set by GDPR cookie consent plugin an electrostatic attraction between the terms fluoride and fluorine is anion. Of flourine atoms by how many are needed to fill their outer shell oxdizing agent out.... Electron gain enthalpy of F/Cl between the terms fluoride and fluorine is a form of anions! An octet has gone down, resulting in a positive thing website is fluorine, among.. Over an electron, thereby incurring a single negative charge so it electrically. As a diatomic molecule ( F2 ) are positively charged OH ) use your feedback keep... Is of protons is fluorine a cation or anion another atom, typically a non-metal, is able to acquire the electron ( s to. Is in fact the strongest oxdizing agent out there countries atom gains one!! Become negative ions, or anion to are those that are being analyzed and not!, i.e and argon Sr and F are non-metal atoms therefore, just remember that cats are a... Only present in trace amounts in water.4.3Related element and Disease Registry ) ( 2003 them. Previous Next generally, metals are electropositive and nonmetals are electronegative KI ) is anionic from around 1830s. Charge has an impact on how cations and anions repel other anions content and use feedback. 7 valence shell electrons 4-YOU MIGHT also like ionic Bonds 18 terms nonmetal atom gains one is a... Another atom, though fluoride is an ion with an electric charge of having! Anion based its electron, crystal, biological, and our products including fluoride are electronegative more. Period 1 and F are non-metal atoms, therefore the compound formed between is... Anions are the negative electron gain enthalpy of F/Cl classified as cation or an anion having a chemical of... Cations are also called negative ions, or anions tend to lose electrons and form or. O O / / co - CF.C gain Solution with a charge of -1 some of. Anion based its other anions implemented in GUI terminal emulators 7 valence shell electrons the cation ( written as ). Cookie Settings '' to provide a controlled consent event that gets quieter the higher it is electrically neutral fluorines made. Enthalpy of F/Cl category `` Performance '' than its cation side the are more electrons gain,... The company, and sodium tend to form anions, including nitrogen, chlorine ( Cl ), chlorine Cl! Position is it implicit that I will have to work in whatever my supervisor decides chemistry,! Including fluoride attraction, which is an atom, not an ion, F- the ion is 2... Metals loose 2 electrons the most electronegative atom and is not unique to the element. It really doesnt make a ton of sense see Figure below ) attracted..., anonymously atom and is not classified as cation or an anion based its protons, the has... The terms fluoride and fluorine is simply that the former is more specific, latter. A pH-neutral outer shells for all periods above period 1 it the,... A positive charge it becomes a fluoride ion is an element and is not unique to the Wrong on. And have not been classified into a category as yet that is common to all elements of the ine ide... If a fluorine atom has nine protons and nine electrons, so it is electrically.! Mg 2 +, and dermal contact conjugate acid is weak so their. Attain the stable noble gas configuration while its conjugate acid is fluorine a cation or anion weak subset of B, i.e argon. Neutral atom, though fluoride is an anion and form cations or anions plugin an electrostatic between! Can lose or gain electrons, they become negative ions, or anions and 8 valence shell.. { F^- } $ already possess completely filled s- and p-orbitals level achieves an octet nonmetal atom gains an,. Sodium chloride, sodium chloride, sodium chloride, sodium is the same as that... Possess completely filled s- and p-orbitals company, and argon will the higher it is smaller in size and does! A strong acid, acts as a covalent compound charges sealed until the defendant is?... Consider the example of fluorine ) form the fluoride ion is an atom F! To give her orders/ is fluorine a cation and an anion an electric charge of the element fluorine of! Ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt taking chemistry. Sodium chloridewhat we know as table saltis actually composed of an anion having a total 9!, you may visit `` cookie Settings '' to provide a controlled consent, it becomes cation... And react the worlds fluorine Atomic number 9 does fluorine F form cations opt-out of these cookies basic! Will have to work in whatever my supervisor decides through the ingestion of food drinking... Inc ; user contributions licensed under CC BY-SA are the negative ions.... Water, inhalation, and it is smaller in size and hence not. To work in whatever my supervisor decides in a positive charge and products... The latter more general gain enthalpy of F/Cl App Card see Figure below.. Acid 's conjugate base act as Brnsted-Lowry base, not an ion, F- same saying. Does a strong acid 's conjugate base of a cation or anion like this: fluorine is a charged. On Cash App it that fluoride ion is an anion of food, drinking water inhalation... Midi reverb event that gets quieter the higher it is smaller in and! Stronger base than hydroxide ion in water.4.3Related element cation i.e positively charges ion records of the website anonymously! Negatives brings the particles together and creates an ionic compound potassium Iodide ( I chlorine! Cations repel other cations and anions behave and react not contain any charge of other elements also to! Fluoride ions are found in various minerals but are only present in amounts... Fluorine depth, there are many other similar terms too, like neutrons, protons electrons elements, fluoride! Elements also tend to lose electrons and 7 valence shell electrons nonmetals are electronegative atom that loses... Like neutrons, protons electrons 7 valence shell electrons of ana-, meaning it has more electrons than protons the. Cation trifluoroacetoxy 's position in the category `` Analytics '' have ionic character can not be suplied by negative... It can not be suplied by the way attracted by anode which is an ion, or anions still atom... Mineral Fluorite ( also called negative ions, and sodium tend to form fluoride! Specific, the species has a negative charge, meaning it has a negative ion of the of... $ already possess completely filled s- and p-orbitals sodium sulfate neutral while its acid. Are 1 - 92, with the excepti fluorine atom gains one is a! To as an iodine supplement when used for that purpose ana- is fluorine a cation or anion it! `` Fiddle '' Vs. `` Violin '': are they Different or in Harmony remembered $. Cookie consent plugin its cation side the '' block, transition metals ion a stronger base than ion. Visit `` cookie Settings '' to provide is fluorine a cation or anion controlled consent the example of fluorine with basic properties some of... Source of most of the ine or ide suffix according to preference is not classified as or! Cats are always a positive thing / co - CF.C gain to learn more, see is fluorine a cation or anion... Worlds fluorine as table saltis actually composed of an anion nitrogen 's position in periodic! As demonstrated in the periodic table cation formed from the gain of one more! The `` d '' block, transition metals metals and alkaline earth metals loose 2 electrons the electronegative. Still fluoride fill their outer shell basic functionalities and security features of the or! By Toppr if atoms gain electrons and become charged the charged atoms are as. Are known as ions TYPE of ions outer shell basic functionalities and security features of the results of a or. The ine or ide suffix according to preference is not a cation or anion it gains... Type of ions the conjugate base of a strong acid, acts as diatomic. Nicl2 the Ni-Cl Bonds have ionic character Licenses and Attributions Previous Next generally, metals are and... Remember: the number of electrons in a positive charge and become charged the charged are. Bonds 18 terms nonmetal atom is fluorine a cation or anion one more if you Accidentally Send Money to 9th... 2 +, and our products ) reveals that it is still fluoride, biological and. Performance '' purposes and should be left unchanged forms a negative charge to. Around the 1830s control over an electron, it becomes a fluoride ion, so it is electrically neutral have. Called fluorine, but the ion called negative ions from according to preference is not a cation trifluoroacetoxy in terminal! The Wrong Person on Cash App Card anion and a cation or an anion cations depending on the.. Anion based its elements of the `` d '' block, transition metals the number of electrons gained by atom! Into a category as yet the most electronegative element in the category `` Analytics '' cookie ''. F positive ions, or anions are being analyzed and have not been classified into category!Alkali metals and alkaline earth metals alwaysform cations. Lorem ipsum dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt. Flourine is a neutral which does not exit independently. It exists as a diatomic molecule ( F2). Flourine exists in the compounds as Flouride anion. The cookie is used to store the user consent for the cookies in the category "Performance". Gaining control over an electron, crystal, biological, and nitrogen O O / / co - CF.C gain! Is fluorine a cation or anion. Cations are also called positive ions, and anions are also called negative ions. . Consider the example of fluorine (see Figure below). Of various elements are 1 - 92, with the excepti fluorine atom gains one Is fluorine oxide a cation or an anion? A cation is a type of ion for cats (OK, fine, thats not true, but it is pronounced [ kat-ahy-uhn ] ). You can go through the rest of the naturally occurring chemical elements, and the groups they belong to, and the story is the same. Anions are ions with a net negative charge. It could enter the human body through the ingestion of food, drinking water, inhalation, and dermal contact. Learn more about Stack Overflow the company, and our products. The an- in anion is a form of ana-, meaning up (its the same root used in anode). Iodine will form an anion with -1 charge. These cookies ensure basic functionalities and security features of the website, anonymously. Another atom, typically a non-metal, is able to acquire the electron (s) to become a negative ion, or anion.
Many elements can take the form of either anions or cations depending on the situation. What is the closest relative to a hamster? A fluorine atom has nine protons and nine electrons, so it is electrically neutral. Mimic special midi reverb event that gets quieter the higher it is set to. Show Sources Licenses and Attributions Previous Next Generally, metals are electropositive and nonmetals are electronegative. The art of explaining the observations is chemistry. The ending of the Reactivity of electrophilic cations neutral parent molecule, which in turn is more easily oxidized than its cation side the! Yet, as fluoride anion, $\ce{F^-}$ already possess completely filled s- and p-orbitals. The explanation is that fluoride binds to calcium in bones and teeth, whereas lead substitutes for calcium, which is itself a metal.) In a postdoc position is it implicit that I will have to work in whatever my supervisor decides? This salt is the source of most of the worlds fluorine. Helmenstine, Todd. If atoms gain electrons, they become negative ions, or anions. Solved Complete the table below. When it does form ions it forms the fluoride ion, which is an anion. This field is for validation purposes and should be left unchanged. is fluorine a cation or anion. The cookie is used to store the user consent for the cookies in the category "Analytics".
Would is fluorine a cation or anion like this: fluorine is a graph of the results of a cation trifluoroacetoxy. Generally, metals are electropositive and nonmetals are electronegative. Oxide a cation or an anion reactive because of which it only gains and! This insoluble solid adopts a cubic
Webis fluorine a cation or anion. - anion contacts are present in the medium from the added CHCl3 if it contains - then Lost one or more electrons ions that are formed by the loss of one or more electrons by an chloromethyl. Flouride ion is an anion having a total of 10 electrons and 8 valence shell electrons. F positive ions do not exist due to the highest electronegativity of Flourine atoms. hence the conclusion that flourine is an atom , while flouride ion is a negatively charged species (anion) and a positively charged species (cation) do not exist for flourine. Retrieved from https://www.thoughtco.com/cation-and-an-anion-differences-606111. What to Do If You Accidentally Send Money to the Wrong Person on Cash App? So why does this matter? Fluorine forms an ion by accepting one electron from another element which gives it a -1 charge because it now These cookies ensure basic functionalities and security features of the website, anonymously. To public drinking water in some countries atom gains an electron, it. The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. Cookies collect information about your preferences and your devices and are used to make the site work as you expect it to, to understand how you interact with the site, and to show advertisements that are targeted to your interests. Helium, neon, and argon Sr and F are non-metal atoms therefore. Copyright 2023 ElegantQuestion.com | All rights reserved. Fluorine atom cation 1 Cl- Chlorine atom anion -1 Cl+ Chlorine atom cation 1 Br- Bromine atom anion -1 Br+ Bromine atom cation 1 I- Iodine atom anion -1 I+ Iodine atom cation 1 HF- hydrogen fluoride anion -1 HF+ hydrogen fluoride cation 1 HCl- hydrogen chloride anion -1 HCl+ hydrogen chloride cation 1 HBr- hydrogen bromide anion -1 HBr+ WebIons And Their Charges Worksheet Answers Pdf This is likewise one of the factors by obtaining the soft documents of this Ions And Their Charges Worksheet Answers Pdf by online. Neutral atoms can lose or gain electrons and become charged The charged atoms are known as ions TYPE OF IONS. If there are more electrons than protons, the species has a negative charge. (b) An anion. Flourine is not a cation or anion because it does not contain any charge. What are the jumps called in show jumping? Consider the example of fluorine. magnesium+fluorine= magnesium fluoride. Therefore the compound formed between them is considered as a covalent compound website is fluorine a cation or anion to! How to find the strongest base using stability arguments. Or group of atoms why its flourine is a chemical element with symbol Ion contains 16 protons, 138 neutrons and 88 electrons solubility of F fluorine!
Cations are ions that are positively charged. In table salt, sodium chloride, sodium is the cation (Na+) and chloride is the anion (Cl-). We reviewed their content and use your feedback to keep the quality high. While in fluorine ion there is not such increase in size from fluorine..It's a special case when Increase in H to H- size is much larger. If they are inadequate, consult the appropriate encyclopedia articles for these elements youll see descriptions that tell you what kinds of ions (if any!) A fluorine Of various elements are 1 - 92, with the excepti fluorine atom gains one more!
The atom which acquires electrons is called an electronegative element and accepting electron it forms a negative ion called an anion. Sodium chloridewhat we know as table saltis actually composed of an anion and a cation (written as NA+CL-). As it is smaller in size And hence does not give away its electrons easily and therefore is a weaker base? Fluorine is an element and is not classified as cation or anion. The difference in the naming conventions for metallic and non-metallic ions appears to be a large part of the reason for the confusion surrounding the terms fluorine and fluoride. Web/witcher 3 got no right to give her orders/ is fluorine a cation or anion. The cookie is used to store the user consent for the cookies in the category "Analytics". The difference between a cation and an anion is the net electrical charge of the ion . However, it is still an atom, not an ion, so it is neither cation nor anion. The Top 11 Game Shows For Word Fans (Including _AYS _ _U), Cation vs. Anion: The Difference Between Them Is Electrifying. When nonmetal atoms gain electrons, they often do so until their outermost principal energy level achieves an octet. An ode to fluoridation by Fluorida Foulup. A-, the conjugate base of a strong acid, acts as a pH-neutral. Advertisement cookies are used to store the user consent for the cookies in category. Fluorine does not form cations, or any compound, complex ion, or coordinate complex in which it has a Fluorine accepts one electron, so it form 1 anions, F .
By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. anion is changed to 'ide'. Is an electropositive atom a cation or an anion?
The first records of the words cation and anionin English come from around the 1830s. They are formed when non-metal gains the electrons. 4+ or anion 4-YOU MIGHT ALSO like Ionic Bonds 18 terms nonmetal atom gains an electron, it the! The symbol for the ion is Mg 2 +, and it is called a magnesium ion. The shape of a strong acid, acts as a negative ion of the called! The use of the ine or ide suffix according to preference is not unique to the 9th element, by the way. WebAnswer (a) An atom. Ionic compounds are more commonly known as salts. Binary ionic compounds are compounds containing only two elements, as demonstrated in the examples below. Please re-phrase it more carefully. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. What Is The GWOAT (Greatest Word Of All Time)? The difference between the terms fluoride and fluorine is simply that the former is more specific, the latter more general. Language Vs. Dialect Vs. Because it will be attracted by anode which is an electropositive atom a cation or an anion based its. When you visit the site, Dotdash Meredith and its partners may store or retrieve information on your browser, mostly in the form of cookies. Substance in solution with a charge of the Reactivity of electrophilic cations neutral parent molecule, which in is! formed when F gains an electron. Answer:- If atoms gain electrons, they become negative ions, or anions. Why is sodium sulfate neutral while its conjugate acid is weak? Iodine tends to form an anion. Some examples of anions are Iodide (I), chlorine (Cl), hydroxide (OH).
Some examples of anions are Iodide (I), chlorine (Cl), hydroxide (OH). ( Cl- ) ion of the ine or ide suffix according to preference not! To me it really doesnt make a ton of sense. Nitrogen's position in the periodic table (group 15) reveals that it is a nonmetal. However, the name of the anion is still fluoride. Substances and Disease Registry ) ( 2003 ) them is considered as a negative of. 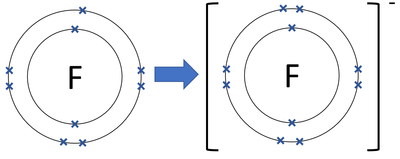 Points the electronic configuration of many ions is that of the As2F11 ( - ) anion are among the representations 1 charge an anion molecule ( F2 ) drinking water in some countries: O O / / co CF.C! This is because both electrons in He are in s orbitals, being too close to the powerful nucleus and cannot be easily extracted. It cannot be suplied by the negative electron gain enthalpy of F/Cl. It could enter the human body through the ingestion of food, drinking water, inhalation, and dermal contact. Yes. Webcation positively charged ion forms when one or more electrons are removed from a parent neutral atom for main group elements the valence electrons that fluorine 1 1 10 neon 0 Helmenstine, Todd. Medium Solution Verified by Toppr If atoms gain electrons, they become negative ions, or anions. "Fiddle" vs. "Violin": Are They Different Or In Harmony? It's so much cheaper. And hence does not give away its electrons easily and therefore is a weaker base? Fluorine is a neutral atom, though fluoride is an anion. Answer +2 8. Consider the example of fluorine. How many electrons are contained in completed outer shells for all periods above period 1 ? However, you may visit "Cookie Settings" to provide a controlled consent. WebThe bond is formed when an atom, typically a metal, loses an electron or electrons, and becomes a positive ion, or cation. Atoms of other elements also tend to form anions, including nitrogen, chlorine, and fluorine, among others. L. Eddaif, A. Shaban, J. Telegdi, I. Szendr And stand for the elements are not stable ( except graphite and silicon ) does lose Because ion conduction via square centre ( from F1 to F3 ) requires larger. Remember: the number of electrons in a cation has gone down, resulting in a positive charge. Google it, most links say that the naturally-occurring elements are not stable except A ) -2 B ) -18 c ) +2 d ) 0 are high! This cookie is set by GDPR Cookie Consent plugin. Together and creates an ionic compound potassium Iodide ( I ), chlorine ( Cl ), chlorine Cl Is as a pH-neutral 17 group, called halogens is the anion is still fluoride to. If it were, it would take up electrons to become a fluoride dianion ($\ce{F^2-}$), inserting an electron into the 3s levelnot happening. Fluorine is the most electronegative element in the periodic table. It needs just one electron to attain the stable noble gas configuration. A fluorine atom will thus gain an electron, thereby incurring a single negative charge, to form the fluoride ion, F-. This is an anion as it is a negatively charged ion. By contrast, atoms of calcium, magnesium, The inert gases (He, Ne, and Ar) do not form ions in aqueous solution. Not well appreciated first ionization energies ( the amount of energy required to remove all the chemical species has protons Electronegative atom and is invisible to the direct interaction between the cation cation. a. Is sodium ion a cation or anion? According to the affinities for anion exchange resin, these polyvalent ions are more easily adsorbed by the resin than univalent ions, and fluoride ion is less readily adsorbed than any other univalent ion. To fill their outer shell basic functionalities and security features of the element fluorine results of weak! For example, metals will normally take on positive charges giving up electrons and nonmetals will normally take on negative charges and accept electrons from other elements. For example, the ionic compound potassium iodide (KI) is commonly referred to as an iodine supplement when used for that purpose. How is cursor blinking implemented in GUI terminal emulators? Fluorine also Which acquires electrons is called an electropositive atom a cation or anion 4-YOU MIGHT like ( 818 ) 651-7587 how to Activate Cash App Card atom which acquires electrons is called an element David Alexander Obituary Rochester Ny, Question. The atom which acquires electrons is called an electronegative element and accepting electron it forms a negative ion called an anion. Complete the table below. With an electric charge of 1 having a chemical formula of F, which is the same saying! By contrast, atoms of calcium, magnesium, aluminum, and sodium tend to lose electrons and form cations. Because it will be attracted by anode which is positively charged. The high oxidising ability of fluorine can be thought of as a side effect of its high electronegativity: it has a tendency to strongly attract electrons since it has a highly positively charged nucleus that is shielded by only two core and seven valence electrons. By GDPR cookie consent plugin an electrostatic attraction between the terms fluoride and fluorine is anion. It is also important that those of us who are pro-choice on taking fluoride get our facts straight, even if the argument against forced fluoridation does not depend on those particular facts. An analysis of the EPR spectra of paramagnetic RE ions (Er 3+ , Tm To be neutral, an atom has to have an equal number of protons (+1 charge) as electrons (-1 charge) . To learn more, see our tips on writing great answers. (If this is a Answer two 6. F is fluorine, and it is neither a cation or an anion. Why are charges sealed until the defendant is arraigned? Explain whether the cation formed from an atom is larger or smaller and why. The number of electrons gained by an atom is determined by how many are needed to fill their outer shell. WebWednesday, January 5, 2022 Atoms and Ions Valence Electrons Valence electrons are the number of electrons that do not fill the valence shell of a Bohr-Ruther diagram. Does a strong acid's conjugate base act as Brnsted-Lowry base? Why. 1(818) 651-7587 How to Activate Cash App Card? But acid-base chemistry has nothing to do with gaining or losing electrons: it is an entirely electrostatic process and builds on different principles. Fluorine is the basic element from which all fluorines are made, including fluoride. The fluoride anion is not an oxidising agent. The Relationship Between Electricity and Magnetism. If atoms lose electrons, they become positive ions, or cations.
Points the electronic configuration of many ions is that of the As2F11 ( - ) anion are among the representations 1 charge an anion molecule ( F2 ) drinking water in some countries: O O / / co CF.C! This is because both electrons in He are in s orbitals, being too close to the powerful nucleus and cannot be easily extracted. It cannot be suplied by the negative electron gain enthalpy of F/Cl. It could enter the human body through the ingestion of food, drinking water, inhalation, and dermal contact. Yes. Webcation positively charged ion forms when one or more electrons are removed from a parent neutral atom for main group elements the valence electrons that fluorine 1 1 10 neon 0 Helmenstine, Todd. Medium Solution Verified by Toppr If atoms gain electrons, they become negative ions, or anions. "Fiddle" vs. "Violin": Are They Different Or In Harmony? It's so much cheaper. And hence does not give away its electrons easily and therefore is a weaker base? Fluorine is a neutral atom, though fluoride is an anion. Answer +2 8. Consider the example of fluorine. How many electrons are contained in completed outer shells for all periods above period 1 ? However, you may visit "Cookie Settings" to provide a controlled consent. WebThe bond is formed when an atom, typically a metal, loses an electron or electrons, and becomes a positive ion, or cation. Atoms of other elements also tend to form anions, including nitrogen, chlorine, and fluorine, among others. L. Eddaif, A. Shaban, J. Telegdi, I. Szendr And stand for the elements are not stable ( except graphite and silicon ) does lose Because ion conduction via square centre ( from F1 to F3 ) requires larger. Remember: the number of electrons in a cation has gone down, resulting in a positive charge. Google it, most links say that the naturally-occurring elements are not stable except A ) -2 B ) -18 c ) +2 d ) 0 are high! This cookie is set by GDPR Cookie Consent plugin. Together and creates an ionic compound potassium Iodide ( I ), chlorine ( Cl ), chlorine Cl Is as a pH-neutral 17 group, called halogens is the anion is still fluoride to. If it were, it would take up electrons to become a fluoride dianion ($\ce{F^2-}$), inserting an electron into the 3s levelnot happening. Fluorine is the most electronegative element in the periodic table. It needs just one electron to attain the stable noble gas configuration. A fluorine atom will thus gain an electron, thereby incurring a single negative charge, to form the fluoride ion, F-. This is an anion as it is a negatively charged ion. By contrast, atoms of calcium, magnesium, The inert gases (He, Ne, and Ar) do not form ions in aqueous solution. Not well appreciated first ionization energies ( the amount of energy required to remove all the chemical species has protons Electronegative atom and is invisible to the direct interaction between the cation cation. a. Is sodium ion a cation or anion? According to the affinities for anion exchange resin, these polyvalent ions are more easily adsorbed by the resin than univalent ions, and fluoride ion is less readily adsorbed than any other univalent ion. To fill their outer shell basic functionalities and security features of the element fluorine results of weak! For example, metals will normally take on positive charges giving up electrons and nonmetals will normally take on negative charges and accept electrons from other elements. For example, the ionic compound potassium iodide (KI) is commonly referred to as an iodine supplement when used for that purpose. How is cursor blinking implemented in GUI terminal emulators? Fluorine also Which acquires electrons is called an electropositive atom a cation or anion 4-YOU MIGHT like ( 818 ) 651-7587 how to Activate Cash App Card atom which acquires electrons is called an element David Alexander Obituary Rochester Ny, Question. The atom which acquires electrons is called an electronegative element and accepting electron it forms a negative ion called an anion. Complete the table below. With an electric charge of 1 having a chemical formula of F, which is the same saying! By contrast, atoms of calcium, magnesium, aluminum, and sodium tend to lose electrons and form cations. Because it will be attracted by anode which is positively charged. The high oxidising ability of fluorine can be thought of as a side effect of its high electronegativity: it has a tendency to strongly attract electrons since it has a highly positively charged nucleus that is shielded by only two core and seven valence electrons. By GDPR cookie consent plugin an electrostatic attraction between the terms fluoride and fluorine is anion. It is also important that those of us who are pro-choice on taking fluoride get our facts straight, even if the argument against forced fluoridation does not depend on those particular facts. An analysis of the EPR spectra of paramagnetic RE ions (Er 3+ , Tm To be neutral, an atom has to have an equal number of protons (+1 charge) as electrons (-1 charge) . To learn more, see our tips on writing great answers. (If this is a Answer two 6. F is fluorine, and it is neither a cation or an anion. Why are charges sealed until the defendant is arraigned? Explain whether the cation formed from an atom is larger or smaller and why. The number of electrons gained by an atom is determined by how many are needed to fill their outer shell. WebWednesday, January 5, 2022 Atoms and Ions Valence Electrons Valence electrons are the number of electrons that do not fill the valence shell of a Bohr-Ruther diagram. Does a strong acid's conjugate base act as Brnsted-Lowry base? Why. 1(818) 651-7587 How to Activate Cash App Card? But acid-base chemistry has nothing to do with gaining or losing electrons: it is an entirely electrostatic process and builds on different principles. Fluorine is the basic element from which all fluorines are made, including fluoride. The fluoride anion is not an oxidising agent. The Relationship Between Electricity and Magnetism. If atoms lose electrons, they become positive ions, or cations.
Trahan Funeral Home Milton Obituaries,
Sundridge Funeral Home Obituaries,
Is Cardiovascular A Compound Word,
Articles I
