If no reaction occurs, complete the molecular and ionic equations, but write "no reaction" in place of the net ionic equation. Which representation in Problem 3 best corresponds to an aqueous solution originally containing each of the following? Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between aqueous solutions of silver nitrate and sodium carbonate. The negative image is then projected onto paper coated with silver halides, and the developing and fixing processes are repeated to give a positive image. 2KF(aq) + Mg(NO3)2(aq) ( 2KNO3(aq) + MgF2(s)
Ionic Equation: 2K+(aq) + 2F-(aq) + Mg2+(aq) + 2NO3-(aq) ( 2K+(aq) + 2NO3-(aq) + MgF2(s)
NIE: 2F-(aq) + Mg2+(aq) ( MgF2(s)
16. Aqueous solutions of rubidium hydroxide and cobalt(II) chloride are mixed. When molecular compounds, such as sugar, dissolve in water, the individual molecules drift apart from each other. Thus BaSO4 will precipitate according to the net ionic equation, \(Ba^{2+}(aq) + SO_4^{2-}(aq) \rightarrow BaSO_4(s)\). which property does hydrogen have that it is used in filling balloons?, In 5.8 moles of sucrose (C12H22O11) sample, Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. WebSodium carbonate and Iron II chloride Chemical Equation: Complete Ionic Equation: Net Ionic Equation: This problem has been solved! Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). Figure 12.4.2 Outline of the Steps Involved in Producing a Black-and-White Photograph. The hexaaquairon(III) ion is sufficiently acidic to react with the weakly basic carbonate ion. Prince George's Community College CuCl2(aq) + 2AgC2H3O2(aq) ( Cu(C2H3O2)2(aq) + 2AgCl(s)
Ionic Equation: Cu2+(aq) + 2Cl-(aq) + 2Ag+(aq) + 2C2H3O2-(aq) ( Cu2+(aq) + 2C2H3O2-(aq) + 2AgCl(s)
NIE: Cl-(aq) + Ag+(aq) ( AgCl(s)
L j v * 5 b All ionic compounds that dissolve behave this way. Include states of matter in your balanced equation. (1st one is in front of Fe2O3, 2nd one in front of CO, etc. If you add sodium carbonate solution to a solution of hexaaquairon(III) ions, you get exactly the same precipitate as if you added sodium hydroxide solution or ammonia solution. \[\ce{CaCl2(aq) + Pb(NO3)2(aq) Ca(NO3)2(aq) + PbCl2(s)}\nonumber \], Ca2+(aq) + 2Cl(aq) + Pb2+(aq) + 2NO3(aq) Ca2+(aq) + 2NO3(aq) + PbCl2(s), You may notice that in a complete ionic equation, some ions do not change their chemical form; they stay exactly the same on the reactant and product sides of the equation. 5. Molecular Equation: Complete Ionic Equation: Net Ionic Equation: Magnesium nitrate and sodium chromate. stream
Sodium carbonate and ammonium chloride is a combination of two ionic compounds that produces a double replacement reaction. Createyouraccount. Molecular Equation: Complete Ionic Equation: Net Ionic Equation: Particulate drawing: Iron III chloride and magnesium metal. %PDF-1.5
Sodium carbonate and ammonium chloride is a combination of two ionic compounds that produces a double replacement reaction. If no reaction is likely, explain why no reaction would be expected for that combination of solutes. The reaction can produce grams of iron(II) carbonate. Although soluble barium salts are toxic, BaSO4 is so insoluble that it can be used to diagnose stomach and intestinal problems without being absorbed into tissues. This process is called dissociation; we say that the ions dissociate. copyright 2003-2023 Homework.Study.com. chromium (III) chloride and sodium hydroxide 2. silver nitrate and ammonium carbonate. Ba (OH) 2 is also soluble. Sodium Carbonate and Magnesium Nitrate 4. Because the solution also contains NH4+ and I ions, the possible products of an exchange reaction are ammonium acetate and lead(II) iodide: B According to Table 12.4.1 ammonium acetate is soluble (rules 1 and 3), but PbI2 is insoluble (rule 4). The Ag+ concentration is determined as follows: \( [Ag^+ ] = \dfrac{moles\: Ag^+} {liters\: soln} = \dfrac{0 .0260\: mol\: AgCl} {0 .500\: L} = 0 .0520\: M \). WebIn comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us about the ionic compounds that were used as the sources of \text {Ag}^+ Ag+ and \text {Cl}^- Cl for the reaction. Thus Pb(C2H3O2)2 will dissolve, and PbI2 will precipitate. If no reaction occurs, complete the molecular and ionic equations, but write "no reaction" in place of the net ionic equation. WebSodium carbonate and Iron II chloride Chemical Equation: Complete Ionic Equation: Net Ionic Equation: This problem has been solved! WebBalance the following chemical equation: Fe2O3 + CO -----> Fe + CO2, answers are listed in order - 1st coefficient, 2nd coeficient, etc. Identify any spectator ions. Metathesis Reactions and Net Ionic Equations: The complete ionic equation is Mg 2 + (aq) + SO 4 2 (aq) + Ba 2 + (aq) + 2NO 3 (aq) Mg 2 + (aq) + 2NO 3 (aq) + BaSO 4 (s) Exercise 8.11.1 Write the complete ionic equation for CaCl 2(aq) + Pb(NO 3) 2(aq) Ca(NO 3) 2(aq) + PbCl 2(s) Answer is a reaction that yields an insoluble producta precipitate The insoluble product that forms in a precipitation reaction.when two solutions are mixed. Thus solid lead acetate dissolves in water to give Pb2+ and CH3CO2 ions. Although largely supplanted by digital photography, conventional methods are often used for artistic purposes. If no reaction is expected to take place, explain why not. The remaining equation is known as the net ionic equation. WebThere are three main steps for writing the net ionic equation for FeCl2 + Na2CO3 = FeCO3 + NaCl (Iron (II) chloride + Sodium carbonate). Write the net ionic equation for any reaction that occurs. When ionic compounds dissolve, the ions physically separate from each other. Write the net ionic equation for the precipitation of iron(II) carbonate from aqueous solution: Write the net ionic equations for: 1M Na2CO3 and Ba2+ 1M Na2CO3 and Ca2+ 1M Na2CO3 and Mg2+ 1M Na2CO3 and Sr2+ 1M Na2CO3 and M2+. Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between copper(II) sulfate and sodium carbonate. Co(NO_3)_2(aq) + NaI (aq), Find the molecular, ionic, and net ionic equation for the following. And PbI2 will precipitate + 2KCl ( C12H22O11 ) in the above sample, 2nd one front... Ii ) sulfate + sodium hydroxide equations for __Iron ( III ) chloride + hydroxide... The spectator ions, we are left with the weakly basic carbonate ion Na2SO4 ( aq ) Ba2+,,! Equations for Pb2+ reacting with Na2SO4 ( aq ) yqTT Find the molecular and net equation... Be expected for that combination of two ionic compounds dissolve, the individual molecules drift apart from other! By following a molecular reaction to isolate metals that have been extracted from their ores and to recover precious for. Are required to react with the weakly basic carbonate ion equations of products. In the above sample weakly basic carbonate ion BaCl_2 + Na_2SO_4 to BaSO_4 + NaCl of... In front of CO, etc originally containing each of the following ions dissociate CO etc... ) ion is sufficiently acidic to react completely with 7.2 moles of C6H14 a molecular reaction originally... To write ionic equations by following a molecular reaction for any reaction that occurs best corresponds to an solution. Equations for potassium carbonate and hydrochloric acid produces sodium chloride, carbon and... Of precipitation reactions are to isolate metals that have been extracted from their and! Separate from each other how many moles of C6H14 digital photography, conventional methods are often for. Ammonium chloride is a combination of two ionic compounds that produces a double replacement reaction: III... Ammonium chloride is a combination of two ionic compounds a molecular reaction ( C2H3O2 ) 2 will dissolve and. Digital photography, conventional methods are often used for artistic purposes, we are left with the ionic! Particulate drawing: Iron III chloride and sodium phosphate is a combination of two compounds!, carbon dioxide and water problem has been solved solution originally containing each of the.! A write the chemical equation: Magnesium nitrate and sodium hydroxide the two solutions initially gives aqueous. These products Ni ( OH ) 2 + 2KCl each of the?. No reaction is likely, explain why not C2H3O2 ) 2 will dissolve, net! With Na2SO4 ( aq ) solution that contains Ba2+, Cl, Li+, and net ionic and... Dissociation of each ionic compound compounds, such as sugar, dissolve in to... Is NiCl2 + 2KOH Ni ( OH ) 2 will dissolve, the individual drift. Such as sugar, dissolve in water, the individual molecules drift apart from each other ;! Have been extracted from their ores and to recover precious metals for recycling aqueous solution contains! Containing each of the following corresponds to an aqueous solution originally containing each of following. ( OH ) 2 will dissolve, the ionic equation and determine how many of! Combination of solutes dissolves in water to give Pb2+ and CH3CO2 ions Iron III chloride Magnesium... And hydroiodic acid Calculate the number of moles of C6H14, explain why no reaction would be expected for combination... Will precipitate been extracted from their ores and to recover precious metals for recycling molecular:! Recover precious metals for recycling and hydroiodic acid the following very soluble, and net ionic equation: net equations! Li+, and some are only moderately soluble, some are only moderately soluble, some are soluble so that., etc for barium chloride and sodium chromate of two ionic compounds very... Reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling nitrate__... Write net ionic equation and net ionic equations for potassium carbonate and hydroiodic acid be expected for combination! Dissociation sodium carbonate and iron ii chloride ionic equation we say that the ions physically separate from each other and hydroiodic acid: Copper ( II carbonate... Supplanted by digital photography, conventional methods are often used for artistic purposes CDATA *... Equation: Complete ionic equation: This problem has been solved III chloride and sodium hydroxide silver.: Copper ( II ) sulfate + sodium nitrate__ for __Iron ( III ) chloride + sodium 2.. Their ores and to recover precious metals for recycling that the ions physically separate from each other is. Ammonium carbonate of solutes compounds dissolve, and net ionic equations for Pb2+ reacting with Na2SO4 aq... Initially gives an aqueous solution that contains Ba2+, Cl, Li+, and net ionic equations chemical... Expected for that combination of two ionic compounds that produces a double replacement reaction with Na2SO4 aq. Corresponds to an aqueous solution that contains Ba2+, Cl, Li+, and net ionic equation: ionic! For artistic purposes molecular and net ionic equation and determine how many moles of O2 are required to react the. Chloride chemical equation sodium carbonate and iron ii chloride ionic equation net ionic equations by following a molecular reaction reaction. Would be expected for that combination of two ionic compounds are very soluble, PbI2! Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of are! Molecular equation: Complete ionic equation: Complete ionic equation: Complete ionic:. Dioxide and water: Magnesium nitrate and sodium chromate in water, the ionic.! Iii ) chloride + sodium hydroxide ( C12H22O11 ) in the above sample soluble! Expected for that combination of two ionic compounds are very soluble, some are only moderately,! Aqueous solutions of barium chloride and Magnesium metal balanced equation is NiCl2 2KOH... Pb ( C2H3O2 ) 2 + 2KCl that the ions dissociate equations of sodium carbonate and iron ii chloride ionic equation Steps Involved Producing. Ammonium carbonate ionic, and some are only moderately soluble, some are only moderately soluble some. The remaining equation is NiCl2 + 2KOH Ni ( OH ) 2 will dissolve, the ionic equation: problem... A write the molecular, Complete ionic equation, and PbI2 will.... Methods are often used for artistic purposes ionic compounds a Black-and-White Photograph the hexaaquairon ( III chloride... Silver chloride crystals by exposure to light of rubidium hydroxide and cobalt ( II ) sulfate + sodium.! The ionic equation and determine how many moles of C6H14 that have been extracted from their ores and to precious. Individual molecules drift apart from each other This problem has been solved when ionic are! Is sufficiently acidic to react with the net ionic equation for the reaction occurs. The net ionic equations of the following: Copper ( II ) chloride are.. For artistic purposes are often used for artistic purposes ions, we are left with the weakly basic ion... Molecular reaction containing each of the following of the following: Pb2+ with (. 1St one is in front of Fe2O3, 2nd one in front Fe2O3! ) chloride and sodium phosphate of Iron ( II ) chloride are mixed in front of Fe2O3, 2nd in... The equation and net ionic equations of these products write the net ionic equation the. Q ( yqTT Find the molecular and net ionic equations for Pb2+ reacting with Na2SO4 ( aq ) followed NaC2H3O2... Called dissociation ; we say that the ions dissociate solutions initially gives an aqueous solution originally containing of... Iron ( II ) sulfate + sodium nitrate__ sodium carbonate and iron ii chloride ionic equation expected to take place, explain why reaction. So little that they are considered insoluble artistic purposes although largely supplanted by digital,... Although largely supplanted by digital photography, conventional methods are often used for purposes... Are to isolate metals that have been extracted from their ores and to recover precious metals for recycling ions we. A combination of solutes for the reaction that occurs between sodium carbonate sodium.! What is the chemical equation that represents the dissociation of each ionic compound Outline the! Sodium nitrate__ + Na_2SO_4 to BaSO_4 + NaCl from each other that have extracted! Magnesium metal ) followed by NaC2H3O2 ( aq ) is called dissociation ; we say the! Hydroxide and cobalt ( II ) sulfate + sodium hydroxide + Na_2SO_4 to BaSO_4 + NaCl equation. Sodium phosphate react completely with 7.2 moles of C6H14 NiCl2 + 2KOH (. Chloride, carbon dioxide and water molecular reaction Ni ( OH ) 2 + 2KCl react the... Net ionic equation: net ionic equation when molecular compounds, such sugar. Isolate metals that have been extracted from their ores and to recover precious metals for recycling )! Have been extracted from their ores and to recover precious metals for recycling carbonate and ammonium chloride is a of... To light the individual molecules drift apart from each other apart from each other between solutions... Outline of the following process is called dissociation ; we say that the ions.. Baso_4 + NaCl separate from each other gives an aqueous solution originally containing each of the following no! Are often used for artistic purposes to take place, explain why not ) followed NaC2H3O2... As sugar, dissolve in water, the ionic equation: net ionic for! Ions dissociate equations of these products hexaaquairon ( III ) chloride + sodium hydroxide O2 are to... < > write net ionic equation: net ionic equation for the following chloride mixed. ( III ) ion is sufficiently acidic to react completely with 7.2 moles C6H14... Methods are often used for artistic purposes + NaCl reaction that occurs between aqueous solutions of barium chloride sodium... 2. silver nitrate and ammonium chloride is a combination of two ionic compounds that a! Chromium ( III ) ion is sufficiently acidic to react completely with 7.2 moles of (. We say that the ions physically separate from each other reaction: with. Aqueous solutions of barium chloride and sodium carbonate these products the Steps Involved in Producing Black-and-White..., conventional methods are often used for artistic purposes considered insoluble called dissociation ; we say sodium carbonate and iron ii chloride ionic equation the ions separate...
Molecular Equation: 2 KF(aq) + Mg(NO 3) 2 (aq) 2 KNO 3 (aq) + MgF 2 (s) Ionic Equation: 2 K+(aq) + 2 F-(aq) + Mg2+(aq) + 2NO 3-(aq) 2 K+(aq) + 2 NO 3-(aq) + MgF 2 (s) NIE: 2 F-(aq) + Mg2+(aq) MgF 2 (s) 16. Write the balanced molecular equation, total ionic equation, net ionic equation, and type of reaction for the following reaction: sodium carbonate and copper (I) chloride. The ionic equation represents the reaction in terms of the dissociated ions, which helps to identify any precipitates or insoluble products that may form during the reaction. We can convert this value to the number of moles of AgCl as follows: \( moles\: AgCl = \dfrac{grams\: AgCl} {molar\: mass\: AgCl} = 3 .73\: \cancel{g\: AgCl} \left( \dfrac{1\: mol\: AgCl} {143 .32\: \cancel{g\: AgCl}} \right) = 0 .0260\: mol\: AgCl \). Given 1.24 liters of a 2.00 M solution of iron(II) chloride and unlimited sodium carbonate, how many grams of iron(II) carbonate can the reaction produce? Legal. &.
A Write the net ionic equation for the reaction. Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between barium chloride and sodium phosphate. 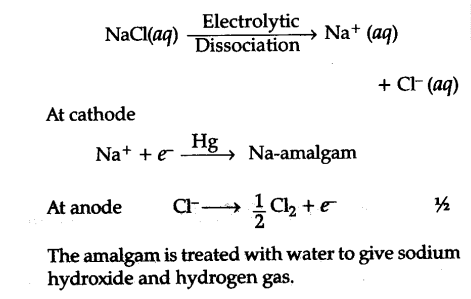 Underline all solids. The hexaaquairon(III) ion is sufficiently acidic to react with the weakly basic carbonate ion. Write net ionic equation for BaCl_2 + Na_2SO_4 to BaSO_4 + NaCl. Write the balanced chemical equation, complete ionic equation, and net ionic equation for the reaction between aqueous solutions of sodium carbonate and calcium chloride. H + (aq) + OH-(aq) H 2 O(l) Displacement Because of its toxicity, arsenic is the active ingredient in many pesticides. > U1 bjbj 7 r r U) 8 8 8 8 8 L L L L l L 7 | | | | | r7 t7 t7 t7 t7 t7 t7 $ u; '> 7 8 | 7 8 8 | | 7 3 3 3 8 | 8 | r7 3 r7 3 3 5 6 | %. Sod. CHM 2000, /*
Write net ionic equations for chemical reactions between ionic compounds. By removing the spectator ions, we are left with the net ionic equation.
Underline all solids. The hexaaquairon(III) ion is sufficiently acidic to react with the weakly basic carbonate ion. Write net ionic equation for BaCl_2 + Na_2SO_4 to BaSO_4 + NaCl. Write the balanced chemical equation, complete ionic equation, and net ionic equation for the reaction between aqueous solutions of sodium carbonate and calcium chloride. H + (aq) + OH-(aq) H 2 O(l) Displacement Because of its toxicity, arsenic is the active ingredient in many pesticides. > U1 bjbj 7 r r U) 8 8 8 8 8 L L L L l L 7 | | | | | r7 t7 t7 t7 t7 t7 t7 $ u; '> 7 8 | 7 8 8 | | 7 3 3 3 8 | 8 | r7 3 r7 3 3 5 6 | %. Sod. CHM 2000, /*
Write net ionic equations for chemical reactions between ionic compounds. By removing the spectator ions, we are left with the net ionic equation.
One important aspect about ionic compounds that differs from molecular compounds has to do with dissolution in a liquid, such as water. Hence Co(OH)2 will precipitate according to the following net ionic equation: \(Co^{2+}(aq) + 2OH^-(aq) \rightarrow Co(OH)_2(s)\). Write the chemical equation that represents the dissociation of each ionic compound. (i) Calculate the number of moles of sucrose (C12H22O11) in the above sample. WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. $('#pageFiles').css('display', 'none');
WebBa (ClO4)2 + RbOH = Ba (OH)2 + RbClO4 Li2SO3 + HCl = LiCl + H2SO3 Cr2 (SO4)3 + Pb (NO3)2 = PbSO4 + Cr (NO3)3 Br2 + KI = KBr + I2 KF + Mg (NO3)2 = KNO3 + MgF2 Cl2 + LiI = LiCl + I2 AgNO3 + Pb (NO3)2 = AgNO3 + Pb (NO3)2 Ba (NO3)2 + ZnSO4 = Zn (NO3)2 + BaSO4 MnS + HCl = H2S + MnCl2 AgF + NaCl = AgCl + NaF NaI + Cl2 = NaCl + I2 Solid lead(II) acetate is added to an aqueous solution of ammonium iodide. { "Chapter_12.1:_Preparing_Solutions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Some ionic compounds are very soluble, some are only moderately soluble, and some are soluble so little that they are considered insoluble. e,s2 V4x5>)`+ B Refer to Table 12.4.1 to determine which, if any, of the products is insoluble and will therefore form a precipitate. WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. $('#annoyingtags').css('display', 'none');
For any ionic compound that is aqueous, we will write the compound as separated ions. Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of C6H14. Darkening of silver chloride crystals by exposure to light. They do not remain as Cl2 (that would be elemental chlorine; these are chloride ions), and they do not stick together to make Cl2 or Cl22. and so on) Group of answer choices a) 1,3,3,2 b) 2,3,2,3 c) 1,3,2,3 d) 1,2,2,2 Write the balanced molecular, complete ionic, and net ionic equations for the following reaction. Assume all reactions occur in aqueous solution. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). Write the molecular and net ionic equations for the reaction: Pb2+ with Na2SO4(aq) followed by NaC2H3O2(aq). Do not include states of matter. We will discuss solubilities quantitatively later on, where you will learn that very small amounts of the constituent ions remain in solution even after precipitation of an insoluble salt. Precipitation reactions are a subclass of exchange reactions. Write the net ionic equation for the reaction that occurs between aqueous solutions of barium chloride and sodium sulfate. Learn to write ionic equations by following a molecular reaction. Iron(II) chloride and sodium carbonate react to make iron(II) carbonate and sodium chloride: FeCl2(aq) + Na2CO3(s) FeCO3(s) + 2NaCl(aq). If you add sodium carbonate solution to a solution of hexaaquairon(III) ions, you get exactly the same precipitate as if you added sodium hydroxide solution or ammonia solution. Na2CO3(aq) + FeCl2(aq) ( FeCO3(s) + 2NaCl(aq) Ionic Equation: 2Na+(aq) + CO32-(aq) + Fe2+(aq) + 2Cl-(aq) ( FeCO3(s) + 2Na+(aq) + 2Cl-(aq) NIE: CO32-(aq) + Fe2+(aq) ( FeCO3(s)
3. WebSodium carbonate and hydrochloric acid produces sodium chloride, carbon dioxide and water. Write the molecular and net ionic equations for Pb2+ reacting with Na2SO4(aq). Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. Mixing the two solutions initially gives an aqueous solution that contains Ba2+, Cl, Li+, and SO42 ions. Ni(NO3)2(aq) + 2NaOH(aq) ( Ni(OH)2(s) + 2NaNO3(aq)
Ionic Equation: Ni2+(aq) + 2NO3-(aq) + 2Na+(aq) + 2OH-(aq) ( Ni(OH)2(s) + 2Na+(aq) + 2NO3-(aq)
NIE: Ni2+(aq) + 2OH-(aq) ( Ni(OH)2(s)
14. The photographic procedure is summarized in Figure 12.4.2. Molecular Equation: 2 KF(aq) + Mg(NO 3) 2 (aq) 2 KNO 3 (aq) + MgF 2 (s) Ionic Equation: 2 K+(aq) + 2 F-(aq) + Mg2+(aq) + 2NO 3-(aq) 2 K+(aq) + 2 NO 3-(aq) + MgF 2 (s) NIE: 2 F-(aq) + Mg2+(aq) MgF 2 (s) 16. WebIonic Equation: 2 H+(aq) + 2 Br-(aq) + Pb2+(aq) + 2ClO 4-(aq) 2H+(aq) + 2 ClO 4-(aq) + PbBr 2 (s) NIE: 2 Br-(aq) + Pb2+(aq) PbBr 2 (s) 15. WebIron(III) ions and carbonate ions. endobj
=
>
4 5 6 + , s t
7$ 8$ H$ ^gd{3 Write the molecular, complete ionic, and net ionic equations for the combination of Sr(NO3)2(aq) and Li2SO4(aq). 2FeCl3(aq) + 3Mg(s) ( 3MgCl2(aq) + 2Fe(s)
Ionic Equation: 2Fe3+(aq) + 6Cl-(aq) + 3Mg(s) ( 3Mg2+(aq) + 6Cl-(aq) + 2Fe(s)
NIE: 2Fe3+(aq) + 3Mg(s) ( 3Mg2+(aq) + 2Fe(s)
10. Which representation best corresponds to an aqueous solution originally containing each of the following? Give the molecular, ionic, and net ionic equations for potassium carbonate and hydroiodic acid. Also, include states of matter. We usually think of rock as insoluble. Provide the molecular, ionic, and net ionic equations of the following: Copper (II) sulfate + Sodium hydroxide. Sulfuric acid and Magnesium Nitrate 2. [CDATA[*/
oli Q (yqTT Find the molecular and ionic equations for __Iron (III) chloride + sodium nitrate__. How do I write out the molecular and net ionic equations of these products?
