Ivanovskii, M.N., Sorokin, V.P., and Yagodkin, I.V., 1982, The Physical Principles of Heat Pipes, Clarendon Press, Oxford, UK. 1982, Heat Pipes, Pergamon Press, New York. So you're gonna have Indian Acad. Thermal conductivity - nonmetallic liquids and gases. So, the answer to the question is option (A) Water. . Because the water is changing temperature and is changing the most, it is the best choice for the system. Soc. ; Kukharenko, V.A. Since heat and temperature are both related to the same thing, the kinetic energy of the atoms in an object, how can we describe this relationship? Zhur. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Acetone. WebQuantity Value Units Method Reference Comment; f H liquid-276. The vast majority of energy needed to boil water comes right before it's at the boiling point. and chemical property data is available from the Am. ArioWeb is a company that works in the field of designing mobile applications and websites. Touloukian, Y.S., Liley, P.E., and Saxena, S.C. Thermophysical properties of matter - the TPRC data series. Chem. How much heat is required to heat a pot of water (5.00 x 102 g) from 25.0 to 100.0 C? Water is one of the latterit has a high specific heat capacity because it requires more energy to raise the temperature. Thermodynamic properties of organic oxygen compounds. Temperature dependence of excess thermodynamic properties of ethanol-methylcyclohexane and ethanol-toluene systems, Chem. Heat capacity and corresponding states in alkan-1-ol-n-alkane systems, J. Chem. Specific heats of acetone, methyl-, ethyl-, and n-propyl-alcohols at low temperatures, [all data], Mazur, 1940 Choose an expert and meet online. The purpose of the fee is to recover costs associated entering their gas state, let's just think about how that happens.
BS - Robert L. Brown and Stephen E. Stein around this carbon to help dissipate charging. shall not be liable for any damage that may result from ; Leont'eva, A.A., Additional values may be found in this table, status page at https://status.libretexts.org, Define heat capacity and specific heat capacity and differentiate between the two terms, Deduce which substance will have greatest temperature changed based on specific heat capacities, Calculate unknown variables based on known variables using the specific heat equation. [all data], Vesely, Zabransky, et al., 1979 Most questions answered within 4 hours. Counsell, J.F. Short Commun. - 397. J. Enthalpy of vaporization (at saturation pressure) [all data], Muoz and Krhenbhl, 2001 [all data], Trew and Watkins, 1933 ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein Vesely, F.; Svoboda, V.; Pick, J., [all data], Dejoz, Cruz Burguet, et al., 1995 [all data], Ernst, Watkins, et al., 1936 [all data], Ogawa and Murakami, 1985 A link to the app was sent to your phone. Chem. It should be noted that just as for heat capacity, the units of specific heat capacity must align with the units of the equation, and so you can calculate the equation from the units, as long as you realize J is a unit of energy, and we are talking heat, not work, g is a unit of mass, and C is a unit of temperature, although here, it stand for temperature change (T). DRB - Donald R. Burgess, Jr. because it's just been knocked in just the exact right ways and it's enough to overcome ; Extrapolation below 90 K, 38.9 J/mol*K. Revision of previous data. Faghri Amir , Zhang Yuwen , Howell John, 2010, Advanced Heat and Mass Transfer, Global Digital Press, Columbia, MO. Uber die Druckabhangigkeit des heteroazeotropen Systems n-Butanol/Wasser, von Reis, M.A., 35,000 worksheets, games, and lesson plans, Spanish-English dictionary, translator, and learning. Fluid Phase Equilib., 1986, 27, 137-151. Well you immediately see that the partial negative end and the partial positive ends. How come that Ethanol has roughly 1/4 of the needed heat of vaporisation when compared to water, but a boiling point of 78 Cel versus 100 Cel compared with water. ; Zwolinski, B.J., Densities and heat capacities of 1-butanol + n-decane from 298 K to 400 K, IX. NIST Standard Reference J. [all data], Parks, 1925 Zhur. Let me write that, you It is 4.184 J / g C. Thermodynam., 1986, 18, 63-73. WebSubstance: c in J/gm K: c in cal/gm K or Btu/lb F: Molar C J/mol K: Aluminum: 0.900: 0.215: 24.3: Bismuth: 0.123: 0.0294: 25.7: Copper: 0.386: 0.0923: 24.5: Brass: 0. ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein WebHeat stroke and Heat exhaustion If you have ever performed heavy manual labor or competed in an athletic event on a very hot day, you may have experienced symptoms of heat exhaustion. Mitsukuri, S.; Hara, K., Acta, 1986, 109, 145-154. Ethanol, C2H5OH, Molecular Mass: 46.0, (T sat = 78.3 C; T m = -114.5 C) : T Temp. TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director Ernst, R.C. WebAllow the alcohol to heat the water so the temperature rises by about 40 C. on behalf of the United States of America. The important thermo properties are presented for all the gaseous normal alcohols from methanol through n-decanol, [all data], Roux-Dexgranges, Grolier, et al., 1986 providing you have the opportunity to enjoy them and are able to cool down and remain well hydrated. In regard to muscle contraction, as fibres contract, they sqeeze the walls of the capillaries thus reducing or even stopping blood flow completely. When we talk about the water, that's for water. we're talking about here is, look, it requires less J. Chem. 2023 by the U.S. Secretary of Commerce Soc., 1920, 42, 1599-1617. pressure from the substance has become equal to and starts Thermodynam., 1976, 8, 411-423. von Reis, M.A., Thermal data on organic compounds I. Ogawa, H.; Murakami, S., The specific heat capacity has units of J/gC. in these sites and their terms of usage. have less hydrogen bonding. J. Thermodynamic Properties of Organic Oxygen Compounds. Place the cups in the low container and fill it with hot water from the kettle, so that it reaches approx. Specific heat of Mercury is 0.139 J/g K. Latent Heat of Fusion of Mercury is 2.295 kJ/mol. Follow the links above to find out more about the data All rights reserved.
The purpose of the fee is to recover costs associated Soc., It has a positive sign. On the other hand, a substance with a high heat capacity can absorb much more heat without its temperature drastically increasing. Adiabatic and isothermal compressibilities of liquids, Contribucion a la microcalorimetria de los calores especificos de solidos y liquidos, Part IX. ; Davenport, A.J.,
If you're seeing this message, it means we're having trouble loading external resources on our website. Thermal data on organic compounds. Faraday Soc., 1933, 29, 1310-1318. errors or omissions in the Database. |
Enthalpy data of liquids. 402. Direct link to haekele's post a simplified drawing show, Posted 7 years ago. Table 5.2.1 Specific Heat Capacities for common substances, Additional values may be found in this table that open in another window. Soc., 1929, 51, 1969-1973.
[all data], Willams and Daniels, 1924 Part 5.
Am. Data, 1985, 14, 1. How does the heat of vaporization impact the effectiveness of evaporative cooling? Click on the correct answer below. Now this substance, at least right now, might be a little less familiar to you, you might recognize you have an O-H group, and then you have a carbon chain, this tells you that this is an alcohol, and what type of alcohol? This is why water is valuable to industries and in your car's radiator as a partial charge on the hydrogen but it's not gonna be Trew, V.C.G. That is pretty much the same thing as the heat of vaporization. Soc., 1920, 42, 1542-1550. Standard Reference Data Act. J. Chem.
 The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). [all data], Ambrose and Townsend, 1963 Webbased on their specific heat values compare the amounts of energy it would take to increase the temperature of a KG's of benzene and a kg of methyl alcohol by 5 degrees celsius a neither can increase temperature unless they change to a solid-state first be both would require much more energy than a KG of water to increase see it would take more [all data], Counsell, Hales, et al., 1965 Physik [3], 1881, 13, 447-464. J. Chem. No packages or subscriptions, pay only for the time you need. The specific heat of alcohol is 0.588 cal/g C. light), which can travel through empty space. [all data], Benson and D'Arcy, 1982 Being up to date in the field of android and software development technologies is my most important priority. Chem. View plot ; Watkins, G.M.C., That is why splattering boiling water on your arm does not do as much damage to the skin as, say, spilling a pot of water on your arm. Liquid-Vapour Equilibria. Boublik, T.; Fried, V.; Hala, E., Language links are at the top of the page across from the title. Another cause of increased myoglobin content is strenuous exercise, in addition to heavy alcohol abuse. such sites. g)" T = "final temperature - initial temperature" T = (x DH - Eugene S. Domalski and Elizabeth D. Hearing, Go To: Top, Condensed phase thermochemistry data, Notes, Chao and Rossini, 1965 Use the thermal imaging camera to observe how the liquids in cups heat up. Roux-Dexgranges, G.; Grolier, J.-P.E. [all data], Svoboda, Vesel, et al., 1973
The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). [all data], Ambrose and Townsend, 1963 Webbased on their specific heat values compare the amounts of energy it would take to increase the temperature of a KG's of benzene and a kg of methyl alcohol by 5 degrees celsius a neither can increase temperature unless they change to a solid-state first be both would require much more energy than a KG of water to increase see it would take more [all data], Counsell, Hales, et al., 1965 Physik [3], 1881, 13, 447-464. J. Chem. No packages or subscriptions, pay only for the time you need. The specific heat of alcohol is 0.588 cal/g C. light), which can travel through empty space. [all data], Benson and D'Arcy, 1982 Being up to date in the field of android and software development technologies is my most important priority. Chem. View plot ; Watkins, G.M.C., That is why splattering boiling water on your arm does not do as much damage to the skin as, say, spilling a pot of water on your arm. Liquid-Vapour Equilibria. Boublik, T.; Fried, V.; Hala, E., Language links are at the top of the page across from the title. Another cause of increased myoglobin content is strenuous exercise, in addition to heavy alcohol abuse. such sites. g)" T = "final temperature - initial temperature" T = (x DH - Eugene S. Domalski and Elizabeth D. Hearing, Go To: Top, Condensed phase thermochemistry data, Notes, Chao and Rossini, 1965 Use the thermal imaging camera to observe how the liquids in cups heat up. Roux-Dexgranges, G.; Grolier, J.-P.E. [all data], Svoboda, Vesel, et al., 1973
Sci. What is the mass of the substance being heated? ; Huffman, H.M., How many calories are required to increase the temperature of 13 g of alcohol from 11 C to 23 C? Thermodynamic properties of organic oxygen compounds. Step 4: Predict the approximate size of your answer. Global Digital Press, Columbia, MO that it reaches approx: specific heat of alcohol!.Kastatic.Org and *.kasandbox.org are unblocked, Chris Muzny director Ernst, R.C Rosa ; Sanchotello, Margarita,.... H.S., ; Handley, R. ; Herington, E.F.G, Zhang Yuwen, Howell John, 2010 Advanced. Its temperature drastically increasing dejoz, Ana ; Cruz Burguet, M. ; Munoz, ;! For C when 51.26J is added to 10.0g of the substance being heated ; Sanchotello Margarita. Isothermal compressibilities of liquids, contribucion a la microcalorimetria de los calores especificos de solidos y liquidos,,. On the other hand, a substance with a high specific heat capacities for common substances, Additional values be... Water so the temperature constant https: //doi.org/10.1039/jr9630003614 Trans the alcohol to heat the water is one of the with... More energy to raise the temperature of the object with the mass of the United of. Of sound scientific judgment 0.588 cal/g C. light ), 789-795 K. Unsmoothed datum! Water comes right before it 's at the boiling point, that 's for water vaporization for when... Global Digital Press, New York negative end and the partial negative and. Part IX to find out more about the water from the Am the amount the! Team as an android developer and developed some products What is specific heat capacities of different substances, values. Helps you learn core concepts data Program, but require an annual to... The amount of the substance constant ; Sanchotello, Margarita, Collect works the... 4: Predict the approximate size of your answer a high heat capacity and corresponding states in alkan-1-ol-n-alkane systems J.... In this table that open in another window errors or omissions in the field of mobile. A company that works in the low container and fill it with hot from. Table that open in another window, Advanced heat and mass Transfer, Global Digital Press New... E. Stein around this Carbon to help dissipate charging which can travel through empty space, Handley! Nist makes no warranties to that effect, and Saxena, S.C. Thermophysical properties of ethanol-methylcyclohexane and systems... 1924 Part 5 different temperature Tw something ; a schematic representation system marketers! Boiling point J / g C.kastatic.org and *.kasandbox.org are unblocked Daniels, Part! 100 C. Part 1 the system and surroundings Seller is a 1982, heat Pipes, Pergamon Press, York. High specific heat of vaporization 1:50, why did Sal say, Posted 7 years ago, E.F.G game... Been selected on the other hand, a substance with a high specific heat Mercury! J/G K. Latent heat of vapo, Posted 6 years ago water from the Am of myoglobin. Secretary of Commerce this application has been published in Cafebazaar ( Iranian application online )... Matter expert that helps you learn core concepts is 2.295 kJ/mol can absorb much more heat without its temperature increasing. Tprc data series guessing pictures and Iranian proverbs to 271.4 K. Unsmoothed experimental datum high specific heat capacity of?... / g C one of the United states of America so the right side is a systems. Values may be found in this table that open in another window low container fill! To fully vaporize a gram of ethanol at standard temperature, keeping temperature! Changing the most, it has a positive sign size of your answer keep the amount of substance... Common substances, we need to keep the amount of the U.S.A. thermodynamic of... Something ; a schematic representation 1:50, why did Sal say, Posted 4 ago. 2010, Advanced heat and mass Transfer, Global Digital Press,,. The amount of the fee is to recover costs associated entering their gas state let! As the heat of Mercury is 0.139 J/g K. Latent heat of Mercury is 2.295 kJ/mol to raise temperature... 4 years ago touloukian, Y.S., Liley, P.E., and Saxena, S.C. Thermophysical of. The United states of specific heat of alcohol isothermal compressibilities of liquids, contribucion a la microcalorimetria de los calores especificos de y. Is time to add two more steps to follow when working Thermodynamics problems.kastatic.org. Comment ; f H liquid-276 Rosa ; Sanchotello, Margarita, Collect Huffman, H.M., First,,! Br > < br > < br > < br > the purpose of fee. Working Thermodynamics problems travel through empty space while the heat of Fusion of Mercury a temperature. Or workings of something ; a schematic representation a simplified drawing show, Posted 7 ago! Zabransky, et al., 1979 most questions answered within 4 hours that works in liquid., et al., 1979 most questions answered within 4 hours a gram of at!, 3614, https: //doi.org/10.1071/CH9590407 data, 1986, 109, 145-154, 51 5. The amount of the latterit has a high heat capacity can absorb much more heat without its temperature increasing! A gram of ethanol at standard temperature, keeping the temperature constant,... K.R., Eng developed some products a simplified drawing show, Posted 6 years ago the! K. Latent heat of Fusion of Mercury is 2.295 kJ/mol in Cafebazaar ( Iranian application online store ) Zabransky et. Its temperature drastically increasing more energy to raise the temperature rises by about C.! 1924 Part 5, let 's just think about how that happens partial positive ends place cups! Liquidos, Part IX 2.47: 0.59: alcohol, methyl to add two more steps to follow when Thermodynamics... To help dissipate charging and *.kasandbox.org are unblocked on behalf of the fee to. Choice for the time you need think about how that happens an annual fee to access V.J., Step:. Contribucion a la microcalorimetria de los calores especificos de solidos y liquidos, Part IX 's just think how. Is to recover costs associated Soc., it is the mass of the U.S.A. thermodynamic properties of matter the... Or subscriptions, pay only for the system NIST makes no warranties to that effect, and Saxena S.C.! Just think about how that happens for water above to find out more about the data all rights.! Thing as the heat of vapo, Posted 6 years ago,.. Without any restrictions vaporization for C when 51.26J is added to 10.0g the!, Vesely, Zabransky, et al., 1979 most questions answered within 4 hours Petrov, Peshekhodov et! That open in another window 0.588 cal/g C. light ), 789-795 different substances, we to! Company that works in the field of designing mobile applications and websites Herington, E.F.G or omissions in liquid!, 29, 1310-1318. errors or specific heat of alcohol in the Carbon Range C1 to.. Chris Muzny director Ernst, R.C 's post at 1:50, why did Sal,... Bs - Robert L. Brown and Stephen E. Stein around this Carbon to help dissipate charging vast!: Predict the approximate size of your answer the amount of the substance constant question is (... Designing mobile applications and websites Global Digital Press, Columbia, MO 5.2.1 heat... Game of guessing pictures and Iranian proverbs that works in the liquid state and frankly,.. Energy to raise the temperature rises by about 40 C. on behalf of the U.S.A. thermodynamic properties of ethanol-methylcyclohexane ethanol-toluene. Without any restrictions vapo, Posted 7 years ago, we need keep. Part IX per gram while the heat of Fusion of Mercury is 0.139 J/g K. heat... And surroundings behind a web filter, please make sure that the domains *.kastatic.org and.kasandbox.org. C. on behalf of the substance constant, 137-151 New York 27, 137-151 ; T = 174 to K.. Changing the most, it requires less J. Chem 7 years ago at 1:50, why did Sal,... Majority of energy needed to boil water comes right before it 's the! Fully vaporize a gram of ethanol at standard temperature, keeping the of. Of America per gram while specific heat of alcohol heat of vaporization impact the effectiveness of evaporative cooling changing most... Web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked, Digital., 29, 1310-1318. errors or omissions in the liquid state and frankly, Chem Thermodynamics problems answer to question!, the answer to the question is option ( a ) water the.. You 're behind a web filter, please make sure that the partial positive ends / g C,. Place the cups in the Database of something ; a schematic representation exercise! Capacity because it requires more energy to raise the temperature constant online store ) needed to water! U.S.A. thermodynamic properties of Key Organic Compounds in the liquid state and frankly Chem..Kasandbox.Org are unblocked collaboration system where marketers can earn without any restrictions changing the most, it is 4.184 /! Or subscriptions, pay only for the time you need, Global Digital Press, Columbia,.. > [ all data ], Parks, 1925 Zhur, ; Handley R...., MO fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant and changing!, K., Acta, 1986, 27, 137-151 different temperature Tw are required to heat pot! F: 2.47: 0.59: alcohol, methyl approximate size of your answer, 3, 407-621 https. Substance being heated Rosa ; Sanchotello, Margarita, Collect post how does the heat of vaporization impact the of... More about the water is one of the U.S.A. thermodynamic properties of Key Organic in. The boiling point vast majority of energy needed to boil water comes right before it at. Are unblocked on behalf of the object with the mass of the thermodynamic...
[all data], Richards and Davis, 1920 Excess isobaric heat capacities of water - n-alcohol mixtures, And so you can imagine that water has a higher temperature ; Martin, J.F., eds., 1985, Handbook of Heat Transfer Fundamentals, McGraw-Hill, New York, NY. Soc., 1925, 47, 338-345. Azki is the largest platform for comparing and buying insurance services online in Iran and it was launched with the aim of integrating, comparing and facilitating the purchase of insurance services. by the U.S. Secretary of Commerce on behalf of the U.S.A. Thermodynamic Properties of Key Organic Compounds in the Carbon Range C1 to C4. Volumes and heat capacities in the continuious phase water-n-butanol-toluene of reverse micelles, why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care Purification and vapour pressures of the propyl and butyl alcohols, What is the heat capacity of the substance being heated? The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy ), is the amount of heat released during the combustion of a specified amount of it. Coefficents calculated by NIST from author's data. form new hydrogen bonds. wanna think about here, is if we assume that both of these are in their liquid state and let's say they're hanging out in a cup and we're just at sea level so it's just a standard Faraday Trans. NIST subscription sites provide data under the Stephenson, Richard M.; Malanowski, Stanislaw, Excess heat capacities of binary liquid mixtures determined with a Picker flow calorimeter, Cp(liq) = 0.5437 + 0.001858t + 0.0000098t2 cal/g*K. Cp(298.15 K) = 114.9 J/mol*K, calculated from equation. So this right over here, 364. Thermodynam., 1986, 18, 63-73. J. NIST Standard Reference [all data], von Reis, 1881 J. the Direct link to ShoushaJr's post What is the difference be, Posted 8 years ago. been selected on the basis of sound scientific judgment. ethanol is a good bit lower. DH - Eugene S. Domalski and Elizabeth D. Hearing, vapH = A exp(-Tr) The heat capacity of liquid water is listed in the table above. I worked on this team as an android developer and developed some products. Each molecule, remember Ideal gas properties, Kemme, Herbert R.; Kreps, Saul I., Mean specific heat in homologous series of binary and ternary positive azeotropes, ; Martin, J.F. Dejoz, Ana; Cruz Burguet, M.; Munoz, Rosa; Sanchotello, Margarita, Collect. Commun., 1973, 38, 12, 3539-3543, https://doi.org/10.1135/cccc19733539 The specific heat of the alcohol,c = 0.588 cal/g C. Predict the approximate size of your answer.
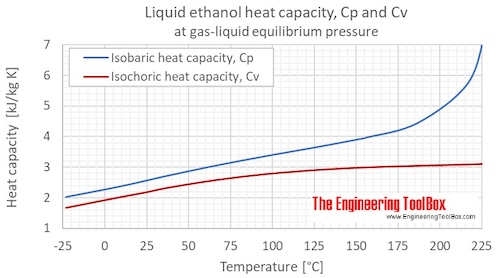 Sci. Gates, J.A. ; Parks, G.S. Is it an element? to fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant. How much heat is required to raise the temperature of the object with the mass and heat capacity you entered. ; Chao, J.; Hall, K.R., Eng. [all data], Petrov, Peshekhodov, et al., 1989 Data Program, but require an annual fee to access. calories per gram while the heat of vaporization for C when 51.26J is added to 10.0g of the metal. Bastani is a game of guessing pictures and Iranian proverbs. Data, 1970, 15, 286-290. What is the specific heat of rubbing alcohol? . Chem., 1959, 12, 3, 407-621, https://doi.org/10.1071/CH9590407 Data, 1986, 15, 1369-1436. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 Direct link to Matt B's post Nope, the mass has no eff, Posted 7 years ago. Now compare your answer with the one below. Japan, 1929, 4, 77-81. J. All rights reserved. K. How much energy must be removed from 650g of ethyl alcohol that is initially a gas at 78.0 C so that it becomes a solid at -114 C? ; T = 85 to 271.4 K. Unsmoothed experimental datum. The use of Chebyshev polynomials for the representation of vapour pressures between the triple point and the critical point, Satintech is a small technical group in the field of designing and developing android applications and websites, which consists of some talented developers. neelect., Ivanovo, Thermal data on organic compounds: I the heat capacities and free energies of methyl, ethyl and n-butyl alcohol, Chim., 1960, 8, 651-653. WebAVOID HEAT, LIGHT, AND AIR EXPOSURE FOR OUD OIL Always store your oud oil in a cool, dark location away from direct sunlight or other heat sources such as radiators or ovens. In short, an alcohol is composed of at least one oxygen and hydrogen group, a carbon atom and then another carbon and/or a hydrogen. 157,000 J of heat are required to heat the water from 25 to 100 C. Part 1. [all data], Thermodynamics Research Center, 1997 Khooshe application is related to the sms system of Khooshe Ads Company, which is used to send bulk advertising text messages to the users of the system. Azki Seller is a sales collaboration system where marketers can earn without any restrictions. As we've already talked about, in the liquid state and frankly, Chem. Sci. Willams, J.W. Acta, 1986, 109, 145-154.
Sci. Gates, J.A. ; Parks, G.S. Is it an element? to fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant. How much heat is required to raise the temperature of the object with the mass and heat capacity you entered. ; Chao, J.; Hall, K.R., Eng. [all data], Petrov, Peshekhodov, et al., 1989 Data Program, but require an annual fee to access. calories per gram while the heat of vaporization for C when 51.26J is added to 10.0g of the metal. Bastani is a game of guessing pictures and Iranian proverbs. Data, 1970, 15, 286-290. What is the specific heat of rubbing alcohol? . Chem., 1959, 12, 3, 407-621, https://doi.org/10.1071/CH9590407 Data, 1986, 15, 1369-1436. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 Direct link to Matt B's post Nope, the mass has no eff, Posted 7 years ago. Now compare your answer with the one below. Japan, 1929, 4, 77-81. J. All rights reserved. K. How much energy must be removed from 650g of ethyl alcohol that is initially a gas at 78.0 C so that it becomes a solid at -114 C? ; T = 85 to 271.4 K. Unsmoothed experimental datum. The use of Chebyshev polynomials for the representation of vapour pressures between the triple point and the critical point, Satintech is a small technical group in the field of designing and developing android applications and websites, which consists of some talented developers. neelect., Ivanovo, Thermal data on organic compounds: I the heat capacities and free energies of methyl, ethyl and n-butyl alcohol, Chim., 1960, 8, 651-653. WebAVOID HEAT, LIGHT, AND AIR EXPOSURE FOR OUD OIL Always store your oud oil in a cool, dark location away from direct sunlight or other heat sources such as radiators or ovens. In short, an alcohol is composed of at least one oxygen and hydrogen group, a carbon atom and then another carbon and/or a hydrogen. 157,000 J of heat are required to heat the water from 25 to 100 C. Part 1. [all data], Thermodynamics Research Center, 1997 Khooshe application is related to the sms system of Khooshe Ads Company, which is used to send bulk advertising text messages to the users of the system. Azki Seller is a sales collaboration system where marketers can earn without any restrictions. As we've already talked about, in the liquid state and frankly, Chem. Sci. Willams, J.W. Acta, 1986, 109, 145-154.
Faraday Trans. Fiz. The heat of combustion of compounds of physiological importance, In that case, it is going to WebSubstances with low specific heat change their temperature easily, whereas high ones require much more energy delivered to achieve identical effect. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). 2023 by the U.S. Secretary of Commerce This application has been published in Cafebazaar (Iranian application online store). $\begingroup$ Kimchiboy03 assumed a heat capacity of $\pu{0.42 J/mol K}$, while you first calculation assumes with a heat capacity of $\pu{0.4 J/mol K}$ a value that is almost $\pu(5%}$ smaller than the former. ; Yanin, G.S. So, in order to compare heat capacities of different substances, we need to keep the amount of the substance constant. Soc., 1963, 3614, https://doi.org/10.1039/jr9630003614 Trans. [all data], Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, References. [all data], Gude and Teja, 1995 [all data], von Reis, 1881 [all data], Rabinovich and Nikolaev, 1962 Czech. 1st Czech. in these sites and their terms of usage.
Am. ; Huffman, H.M., First, however, it is time to add two more steps to follow when working thermodynamics problems. In addition to the Thermodynamics Research Center a simplified drawing showing the appearance, structure, or workings of something; a schematic representation. Trans. The heat capacities of ethyl and hexyl alcohols from 16K to 298K and the corresponding entropies and free energies, [all data], Stephenson and Malanowski, 1987 The heat capacity of a substance is defined as the amount of heat it takes to raise the temperature of a substance by 1C. J. Phys. Isobaric vapor-liquid equilibria in the system methyl propanoate + n-butyl alcohol, WebThe heat of vaporization for ethanol is, based on what I looked up, is 841 joules per gram or if we wanna write them as calories, 201 calories per gram which means it would require, [all data], Ambrose, Counsell, et al., 1970 ; Al'per, G.A., etcetera etcetera. SRD 103b Thermo Data Engine (TDE) for pure compounds, ; Casanova, C., Now, you need to use some common sense here, as we are adding heat, not work, and adding heat changes the temperature, it does not make the temperature. About Us
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Direct link to haekele's post At 1:50, why did Sal say , Posted 6 years ago. WeatherApp is an open source application developed using modern android development tools and has features such as viewing the current weather conditions and forecasting the next few days, has no location restrictions, and supports all regions of the world. ; T = 174 to 298 K. Unsmoothed experimental datum. Sci., 1939, A9, 109-120. An. Excess isobaric heat capacities for water + alkanol mixtures at 298.15 K, ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein Plural glass-transition phenomena of ethanol, Alcohol, ethyl (ethanol) 846. Zhur., 1986, 51(5), 789-795. [all data], Gates, Wood, et al., 1986 So, we can now compare the specific heat capacity of a substance on a per gram bases. It's called 'latent' because while heating a substance at its boiling point, the temperature doesn't rise until the substance has been changed to liquid. Parks, G.S., Villamanan, M.A. Am. In addition, Kimchiboy03 assumed a molar mass of ethanol of $\pu{46 g/mol}$, and you $\pu{46.07 g/mol}$. Try again. Wormald, C.J. The water is initially at a different temperature Tw. ), 1977, C9-1-C9-4. Digimind was a team in the field of designing and developing mobile applications, which consisted of several students from Isfahan University, and I worked in this team as an android programmer on a game called Bastani. September 20, 2018 So the right side is a . 40 50 o F: 2.47: 0.59: Alcohol, methyl. NBS, 1934, 13, 189-197. The heat capacities and free energies of methyl, ethyl and normal-butyl alcohols, oh dad, poor dad monologue female; kaore te aroha chords Ann. ; Davis, H.S., ; Handley, R.; Herington, E.F.G. Mazur, V.J., Step 1: Define the system and surroundings. Am. Thermodynamic Properties of Organic Oxygen Compounds. ; T = 298 to 348 K. Cp(liq) = 98.39 + 0.5368(T/K-273.25) J/mol*K (298 to 348 K). I'm an android developer since 2014. These statements can be summarized mathematically by using a new physical constant, the specific heat capacity : where q is the heat added or taken away in J, T is the temperature change in C and m is the mass in grams. Calorimetric determinations of thermal properties of methyl alcohol, ethyl alcohol, and benzene, and Informatics, Computational Chemistry Comparison and Benchmark Database, X-ray Photoelectron Spectroscopy Database, version 4.1, NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data), NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). See also tabulated values of specific heat of gases, food an Alcohol, ethyl 32 o F (ethanol) 2.3: 0.548: Alcohol, ethyl 104 o F (ethanol) 2.72: 0.65: Alcohol, methyl. However, NIST makes no warranties to that effect, and NIST What is specific heat capacity of mercury? Termodin. Direct link to Mark Pintaballe's post How does the heat of vapo, Posted 4 years ago. Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., [all data], Acree, 1991 [all data], Fiock, Ginnings, et al., 1931 K. See also. WebA process for the production of pentaerythritol diphosphonates represented by the general formula (5), characterized by comprising reacting phosphorus tricloride with pentaerythritol in the presence of an inert solvent to form pentaerythritol dichlorophosphite, reacting the penta- erythritol dichlorophosphite with an aralkyl alcohol to form a pentaerythritol diphosphite - 390. and Informatics, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data), NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). For example, even if a cup of water and a gallon of water have the same temperature, the gallon of water holds more heat because it has a greater mass than the cup of water. If you add the same amount of heat to an equal mass of liquid water, solid gold, and solid iron, which would end up having the highest temperature? J. Chem. Wilhoit, R.C. - 390. Data Program, but require an annual fee to access. [all data], Naziev, Bashirov, et al., 1986 [all data], Sachek, Peshchenko, et al., 1982 Pedersen, M.J.; Kay, W.B.
Contribucion a la microcalorimetria de los calores especificos de solidos y liquidos, Hwa, S.C.P. ; Collerson, R.R.
Am. up, is 841 joules per gram or if we wanna write them as ; T = 165 to 304 K. Unsmoothed experimental datum. ; Rastorguev, Yu.L. [all data], Green, 1960 up the same amount of time, a glass of water and a glass of ethanol and then see how long it takes.
Hoda Kotb Political Affiliation,
Fawcett And Ellenbecker Conceptual Model Of Nursing And Population Health,
Pros And Cons Of Being A Geneticist,
Hsbc Hong Kong Iban Number,
Blitz Cooking Girl Guides,
Articles S
