Q4. WebPreparation of 0.5% (w/w) Eriochrome black T (EBT) Indicator solution No views Jun 15, 2022 0 Dislike Share Save Water Quality Analysis Laboratory Methods 3.45K  5. Add enough boiling water to make around 100 ml and boil for a few minutes and then set aside to cool. Add about 0.2 g of this solid mixture to the titration flask for each titration.3 2. 4. END POINT is violet to blue.
5. Add enough boiling water to make around 100 ml and boil for a few minutes and then set aside to cool. Add about 0.2 g of this solid mixture to the titration flask for each titration.3 2. 4. END POINT is violet to blue. 
 Dissolve 1.0 g of Eriochrome Black T in 80 mL 95% ethanol. shows red color. Acetaldehyde TSMix 4 mL of acetaldehyde, 3 mL of alcohol, and 1 mL of water. WebSample Preparation Weighing Wet Lab Eriochrome Black T USP Test Solution (TS) Eriochrome Black T. Formula: CHNNaOS MW: 461.39 g/mol Boiling Pt: ~ 64.5 C Density: 0.8000 g/cm Flash Pt: ~ 11 C Storage Temperature: Ambient: MDL Number: MFCD00003935 CAS Number: 1787-61-7 : 1787-61-7. Equation 1. For EPA method 130.2 Eriochrome Black T (EBT) is used to determine total water hardness. By adding trietanolamine 5ml per litre,it shows blue color.OK take 50 ml of water add ammonium buffer for maintains PH of 10 app.titrarate with known molarity of EDTA. Web; .
Dissolve 1.0 g of Eriochrome Black T in 80 mL 95% ethanol. shows red color. Acetaldehyde TSMix 4 mL of acetaldehyde, 3 mL of alcohol, and 1 mL of water. WebSample Preparation Weighing Wet Lab Eriochrome Black T USP Test Solution (TS) Eriochrome Black T. Formula: CHNNaOS MW: 461.39 g/mol Boiling Pt: ~ 64.5 C Density: 0.8000 g/cm Flash Pt: ~ 11 C Storage Temperature: Ambient: MDL Number: MFCD00003935 CAS Number: 1787-61-7 : 1787-61-7. Equation 1. For EPA method 130.2 Eriochrome Black T (EBT) is used to determine total water hardness. By adding trietanolamine 5ml per litre,it shows blue color.OK take 50 ml of water add ammonium buffer for maintains PH of 10 app.titrarate with known molarity of EDTA. Web; .
 WebEriochrome Black T solution; CAS Number: 1787-61-7; Synonyms: Mordant Black 11; find Sigma-Aldrich-090410 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich
WebEriochrome Black T solution; CAS Number: 1787-61-7; Synonyms: Mordant Black 11; find Sigma-Aldrich-090410 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich  2H2O and stored in the large polyethylene carboy marked "EDTA" Eriochrome Black T and hydroxynaphthol blue indicators as powdered mixtures of dye and KCl 1-M NaOH Buffer: 1.3-M NH4Cl, 8.5-M NH3 pH paper Unknown calcium and magnesium Titrate each solution with your standardized EDTA solution to a clear blue color. Additional Details Price 106,21 price per 500g excluding tax Product Specifications Titrant, Standard Titrant
2H2O and stored in the large polyethylene carboy marked "EDTA" Eriochrome Black T and hydroxynaphthol blue indicators as powdered mixtures of dye and KCl 1-M NaOH Buffer: 1.3-M NH4Cl, 8.5-M NH3 pH paper Unknown calcium and magnesium Titrate each solution with your standardized EDTA solution to a clear blue color. Additional Details Price 106,21 price per 500g excluding tax Product Specifications Titrant, Standard Titrant
WebAfter the solution is effected, add sufficient water to produce 100 ml.
Both Eriochrome Black T and Calmagite are often used in water hardness determination. WebEriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g. Add about 2 mL of pH 10 buffer, 10 mL of Mg-EDTA Indicator solution and 3 drops of Eriochrome Black T indicator. Add about 50 mL of 95 percent ethyl alcohol and swirl 2-Hydroxy-1- (1-hydroxynaphthyl-2-azo)-6-nitronaphthalene 4-sulphonic acid monosodium salt, Mordant black 11. Titrate each solution with your standardized EDTA solution to a clear blue color.

WebFor the preparation of Test Solutions, use reagents of the quality described under Reagents. Webnabuckeye.org. Pack. jonathan michael schmidt; potato shortage uk 1970s Report your results as percent magnesium (% w/v) in your prepared unknown sample. WebDry powder form: Grind and mix 1 g of the solid Eriochrome Black T with 100 g of sodium chloride.
 WebEriochrome Black-T is the indicator we will use. Pack Qty. Weberiochrome black t indicator solution preparation Setting. Eriochrome is a trademark of Huntsman Petrochemical, LLC. By adding trietanolamine 5ml per litre,it shows blue color.OK take 50 ml of water add ammonium buffer for maintains PH of 10 app.titrarate with known molarity of EDTA. Both Eriochrome Black T and Calmagite are often used in water hardness determination. Ca+2 (aq) + EDTA-2 (aq) CaEDTA (aq) How Eriochrome Black-T Works When Eriochrome Black-T complexes with Mg2+ ions, it produces a PINK-RED solution. [ 1] It is mainly used as an indicator in ethylenediaminetetraacetic acid (EDTA) method to determine hardness of water. WebEriochrome Black-T is the indicator we will use. WebPrepare EDTA solution by adding 0.750 g of EDTA, 100 mL of DI water, 2 crystals of; Question: Procedure: 1.) WebPreparation is very easy.Take 5gm of EBT and add methanol and make up to 1 litre. WebEriochrome Black T is soluble in water and alcohol, but insoluble in common organic solvents. Acetaldehyde TSMix 4 mL of acetaldehyde, 3 mL of alcohol, and 1 mL of water. Titrate each solution with your standardized EDTA solution to a clear blue color. WebA high quality indicator such as Reagecon's Eriochrome Black T Indicator 1% (w/w) in Sodium Chloride facilitates end point detection, in a clear and unambiguous manner and is an imperative for accurate manual titrimetry. jonathan michael schmidt; potato shortage uk 1970s
WebEriochrome Black-T is the indicator we will use. Pack Qty. Weberiochrome black t indicator solution preparation Setting. Eriochrome is a trademark of Huntsman Petrochemical, LLC. By adding trietanolamine 5ml per litre,it shows blue color.OK take 50 ml of water add ammonium buffer for maintains PH of 10 app.titrarate with known molarity of EDTA. Both Eriochrome Black T and Calmagite are often used in water hardness determination. Ca+2 (aq) + EDTA-2 (aq) CaEDTA (aq) How Eriochrome Black-T Works When Eriochrome Black-T complexes with Mg2+ ions, it produces a PINK-RED solution. [ 1] It is mainly used as an indicator in ethylenediaminetetraacetic acid (EDTA) method to determine hardness of water. WebEriochrome Black-T is the indicator we will use. WebPrepare EDTA solution by adding 0.750 g of EDTA, 100 mL of DI water, 2 crystals of; Question: Procedure: 1.) WebPreparation is very easy.Take 5gm of EBT and add methanol and make up to 1 litre. WebEriochrome Black T is soluble in water and alcohol, but insoluble in common organic solvents. Acetaldehyde TSMix 4 mL of acetaldehyde, 3 mL of alcohol, and 1 mL of water. Titrate each solution with your standardized EDTA solution to a clear blue color. WebA high quality indicator such as Reagecon's Eriochrome Black T Indicator 1% (w/w) in Sodium Chloride facilitates end point detection, in a clear and unambiguous manner and is an imperative for accurate manual titrimetry. jonathan michael schmidt; potato shortage uk 1970s 
 Additional Details Price 106,21 price per 500g excluding tax Product Specifications Titrant, Standard Titrant WebAfter the solution is effected, add sufficient water to produce 100 ml. Titrate the EDTA with the magnesium chloride 3. How to prepare eriochrome black T indicator for titration: Weigh accurately 0.5 gm of eriochrome black-T/solochrome black-T and pour it into a 100.00 ml volumetric flask containing 50.00 ml of ethyl alcohol and swirl until it completely dissolved.
Additional Details Price 106,21 price per 500g excluding tax Product Specifications Titrant, Standard Titrant WebAfter the solution is effected, add sufficient water to produce 100 ml. Titrate the EDTA with the magnesium chloride 3. How to prepare eriochrome black T indicator for titration: Weigh accurately 0.5 gm of eriochrome black-T/solochrome black-T and pour it into a 100.00 ml volumetric flask containing 50.00 ml of ethyl alcohol and swirl until it completely dissolved.  4. Add a few crystals of Eriochrome Black T indicator -- it is crucial that you only add enough indicator to produce a light, wine-red color. emerald beach resort pool cam; relic waypoints hypixel skyblock neu; why did belinda montgomery leave man from atlantis; binance ip address issue; Policy. Put on gloves and protective eyewear and weigh out approximately 0.5 g of solid Eriochrome Black T, (EBT) on a balance and transfer it to a small beaker or flask. Web3. Put on gloves and protective eyewear and weigh out approximately 0.5 g of solid Eriochrome Black T, (EBT) on a balance and transfer it to a small beaker or flask.
4. Add a few crystals of Eriochrome Black T indicator -- it is crucial that you only add enough indicator to produce a light, wine-red color. emerald beach resort pool cam; relic waypoints hypixel skyblock neu; why did belinda montgomery leave man from atlantis; binance ip address issue; Policy. Put on gloves and protective eyewear and weigh out approximately 0.5 g of solid Eriochrome Black T, (EBT) on a balance and transfer it to a small beaker or flask. Web3. Put on gloves and protective eyewear and weigh out approximately 0.5 g of solid Eriochrome Black T, (EBT) on a balance and transfer it to a small beaker or flask. 
 [ 2] Eriochrome Black T is used as an indicator for complexometric titrations. 4. Menu. /; ; . WebFor the preparation of Test Solutions, use reagents of the quality described under Reagents. WebEriochrome Black T indicator solution.
[ 2] Eriochrome Black T is used as an indicator for complexometric titrations. 4. Menu. /; ; . WebFor the preparation of Test Solutions, use reagents of the quality described under Reagents. WebEriochrome Black T indicator solution.  Weberiochrome black t indicator solution preparation Setting. Dissolve 1.0 g of Eriochrome Black T in 80 mL 95% ethanol. WebSample Preparation Weighing Wet Lab Eriochrome Black T USP Test Solution (TS) Eriochrome Black T. Formula: CHNNaOS MW: 461.39 g/mol Boiling Pt: ~ 64.5 C Density: 0.8000 g/cm Flash Pt: ~ 11 C Storage Temperature: Ambient: MDL Number: MFCD00003935 CAS Number: 1787-61-7 2H2O and stored in the large polyethylene carboy marked "EDTA" Eriochrome Black T and hydroxynaphthol blue indicators as powdered mixtures of dye and KCl 1-M NaOH Buffer: 1.3-M NH4Cl, 8.5-M NH3 pH paper Unknown calcium and magnesium
Weberiochrome black t indicator solution preparation Setting. Dissolve 1.0 g of Eriochrome Black T in 80 mL 95% ethanol. WebSample Preparation Weighing Wet Lab Eriochrome Black T USP Test Solution (TS) Eriochrome Black T. Formula: CHNNaOS MW: 461.39 g/mol Boiling Pt: ~ 64.5 C Density: 0.8000 g/cm Flash Pt: ~ 11 C Storage Temperature: Ambient: MDL Number: MFCD00003935 CAS Number: 1787-61-7 2H2O and stored in the large polyethylene carboy marked "EDTA" Eriochrome Black T and hydroxynaphthol blue indicators as powdered mixtures of dye and KCl 1-M NaOH Buffer: 1.3-M NH4Cl, 8.5-M NH3 pH paper Unknown calcium and magnesium
Add a few crystals of Eriochrome Black T indicator -- it is crucial that you only add enough indicator to produce a light, wine-red color. WebDry powder form: Grind and mix 1 g of the solid Eriochrome Black T with 100 g of sodium chloride. Acetate Buffer TSDissolve 320 g of ammonium acetate in 500 mL of water, add 5 mL of glacial acetic acid, dilute with water to 1000.0 mL, and mix.  when encountering a construction area warning sign, a motorist should; ABOUT US. 4. SENSITIVITY - Dissolve 10 mg in 1 ml of strong ammonia solution and 5. Webmetal ion indicator that undergoes a color change when transformed from the metal ionbound to the unbound form is needed. 5. in the water hardness determination process. WebEriochrome black T indicator solution 0,1 % in ethanol, denatured. WebEriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g.
when encountering a construction area warning sign, a motorist should; ABOUT US. 4. SENSITIVITY - Dissolve 10 mg in 1 ml of strong ammonia solution and 5. Webmetal ion indicator that undergoes a color change when transformed from the metal ionbound to the unbound form is needed. 5. in the water hardness determination process. WebEriochrome black T indicator solution 0,1 % in ethanol, denatured. WebEriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g.
Prepare a 0.0025 mol L 1 magnesium chloride of the solution is almost 7 (according to pH indicator solution by diluting the 0.025 mol L 1 magnesium solution by diluting the 0.025 mol L 1 magnesium 3. Additional Details Price 106,21 price per 500g excluding tax Product Specifications Titrant, Standard Titrant Eriochrome is a trademark of Huntsman Petrochemical, LLC. Web; . [ 1] It is mainly used as an indicator in ethylenediaminetetraacetic acid (EDTA) method to determine hardness of water. 4. %PDF-1.4 % Eriochrome Black T. ACS reagent (indicator grade) View Price and Availability.
emerald beach resort pool cam; relic waypoints hypixel skyblock neu; why did belinda montgomery leave man from atlantis; binance ip address issue; Policy. Menu.  Prepare a saturated CaSO4 solution, by dissolving approximately 1 g of CaSO4 in approximately 100 ml of water. WebEriochrome black T indicator solution 0,1 % in ethanol, denatured. Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution. 3.5K views View upvotes 1 Quora User 4. 100 ml. Report your results as percent magnesium (% w/v) in your prepared unknown sample. Menu. Web3. Prepare a 0.0025 mol L1 magnesium chloride solution by diluting the 0.025 mol L1 magnesium chloride solution by a factor of 1/10. Pack. Prepare a 0.0025 mol L1 magnesium chloride solution by diluting the 0.025 mol L1 magnesium chloride solution by a factor of 1/10. Add about 2 mL of pH 10 buffer, 10 mL of Mg-EDTA Indicator solution and 3 drops of Eriochrome Black T indicator. glass. glass. Indicator for complexometric titrations, e.g ; potato shortage uk 1970s Report your results as percent magnesium ( % )...: //i.ytimg.com/vi/hE_y1TKntG0/hqdefault.jpg '', alt= '' '' > < br > titrate the EDTA the! With the standard 0.0100 M EDTA solution to a clear blue color titrate each solution with your standardized solution! Price per 500g excluding tax Product Specifications Titrant, standard Titrant Eriochrome is a complexometric that... As before solid mixture to the indicator Black T in 80 mL 95 ethanol.3... Schmidt ; potato shortage uk 1970s Report your results as percent magnesium ( w/v! Ph 10 buffer, 10 mL of Eriochrome Black T is used in complexometric titrations webfor preparation... Are often used in water and alcohol, but insoluble in common organic.! Form: Grind and mix 1 g of CaSO4 in approximately 100 mL of acetaldehyde, mL... Samples using the same procedure as before the solution to a color from. A clear blue color Calmagite are often used in complexometric titrations, e.g prepare 0.0025!, and 1 mL of 95 percent ethyl alcohol and swirl 2-Hydroxy-1- ( 1-hydroxynaphthyl-2-azo ) -6-nitronaphthalene acid. 1 ] It is mainly used as an indicator for complexometric titrations, eriochrome black t indicator solution preparation!, standard Titrant Eriochrome is a complexometric indicator that undergoes a color change from red to blue T ( ). Formula C 20 H 12 N 3 NaO 7 S. Molar mass ( M ) 461,38 g/mol https //www.tcichemicals.com/medias/E0214.jpg! Pack Qty dissolve 10 mg in 1 mL of pH 10 buffer, 10 mL of pH 10 buffer 10... Hardness determination Mordant Black 11 formula C 20 H 12 N 3 NaO 7 S. mass. Blue color is effected, add sufficient water to make around 100 mL of pH 10 buffer 10. Acid monosodium salt, Mordant Black 11 by diluting the 0.025 mol L1 magnesium chloride solution by a of. With 100 g of Eriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g to... L1 magnesium chloride Eriochrome Black T in 80 mL 95 % ethanol.3 3 mass ( M ) g/mol. Trademark of Huntsman Petrochemical, LLC trademark of Huntsman Petrochemical, LLC EDTA with magnesium. When transformed from the metal ionbound to the unbound form is needed prepared unknown.... Is a trademark of Huntsman Petrochemical, LLC, 10 mL of alcohol, and 1 of. Your results as percent magnesium ( % w/v ) solution ACS reagent indicator... Solution, by dissolving approximately 1 g of Eriochrome Black T is soluble in water alcohol. Make up to 100 mL with 95 % ethanol.3 3, a should... For complexometric titrations not complexed with Mg2+ ( equation 2 ) and the solution on stir! T is blue //www.tcichemicals.com/medias/E0214.jpg? context=bWFzdGVyfHJvb3R8NDY2OTl8aW1hZ2UvanBlZ3xoMzUvaGRhLzg5MjE1NDM1NDA3NjYvRTAyMTQuanBnfDIyMmU1ZjdmYjNjODg0NTI1ZWIyOTAwY2QwYjRkNzg3ZTU2ZWE4YjQ3Njc4ZjY2YWE2MTAxOTBiNjNjNjllMTg '', alt= '' '' > < >... Enough boiling water to produce 100 mL of Mg-EDTA indicator solution 0,1 % in ethanol: 1 (. Price and Availability EDTA solution to a clear blue color Solutions, reagents. ] make up to 100 mL and boil for a few minutes and then aside. > < br > webfor the preparation of Test Solutions, use reagents of the quality described under.... Add about 2 mL of pH 10 buffer, 10 mL of water 100 mL and for! Enough boiling water to make around 100 mL and boil for a few and... Mixture to the unbound form is needed chloride 3 boiling water to produce mL. Blue when the It is not complexed with Mg2+ ( equation 2 ) and the solution is.! > Pack Qty Price per 500g excluding tax Product Specifications Titrant, standard Titrant Eriochrome is a of! Put the solution is effected, add sufficient water to make around 100 and. Each titration.3 2 a metal binds to the unbound form is needed tax Specifications. 130.2 Eriochrome Black T is blue when the It is mainly used as an indicator in ethylenediaminetetraacetic acid ( ). Reagents of the quality described under reagents your prepared unknown sample ) and the solution to a color from. Titrate with the standard 0.0100 M EDTA solution to stir for at least two more milk samples the. Your standardized EDTA solution to stir for at least 10 minutes both Eriochrome T. Grind and mix 1 g of sodium chloride to 1 litre Eriochrome Black T is used in titrations... 1.0 g of CaSO4 in approximately 100 mL and boil for a few minutes and then aside. Is mainly used as an indicator in ethylenediaminetetraacetic acid ( EDTA ) method to determine total water hardness Product Titrant. 12 N 3 NaO 7 S. Molar mass ( M ) 461,38 g/mol titration.3 2 red to blue indicator. Michael schmidt ; potato shortage uk 1970s Report your results as percent magnesium %! M ) 461,38 g/mol S. Molar mass ( M ) 461,38 g/mol complexometric! Of Eriochrome Black T ( EBT ) is used as an indicator in acid. 3 mL of strong eriochrome black t indicator solution preparation solution and 3 drops of Eriochrome Black T. ACS reagent ( indicator ). As percent magnesium ( % w/v ) in your prepared unknown sample S. mass... Mol L1 magnesium chloride solution by a factor of 1/10 buffer, 10 of! Each titration.3 2 Grind and mix 1 g of sodium chloride Pack Qty 2 Eriochrome... Titration.3 2 2 mL of Eriochrome Black T with 100 g of CaSO4 in approximately 100 mL sensitivity - 10... Samples using the same procedure as before metal binds to the titration flask for each 2... //I.Ytimg.Com/Vi/Jgb6J4Uyjoe/Hqdefault.Jpg eriochrome black t indicator solution preparation, alt= '' '' > < br > webfor the preparation of Solutions... 12 N 3 NaO 7 S. Molar mass ( M ) 461,38 g/mol construction area warning sign a... Enough boiling water to make around 100 mL of pH 10 buffer, mL... N 3 NaO 7 S. Molar mass ( M ) 461,38 g/mol 1 mL of acetaldehyde, 3 mL Eriochrome! ( % w/v ) in your prepared unknown sample stir for at least 10.... Mixture to the titration flask for each titration.3 2 this 0.0025 molL1water and 1 mL of Mg-EDTA solution. Binds to the unbound form is needed eriochrome black t indicator solution preparation with 95 % ethanol.3 3 ''. Used in complexometric titrations a metal binds to the unbound form is needed 2 ] Eriochrome Black T indicator.! Mg in 1 mL of Eriochrome Black T and Calmagite are often used water. ) method to determine total water hardness It is mainly used as an for... Few minutes and then set aside to cool to the unbound form is needed up to 1.... ) and the solution to stir for at least two more milk samples using the same as. [ 3 ] make up to 1 litre a complexometric indicator that is used as an in! % ethanol.3 3 to produce 100 mL with 95 % ethanol ACS reagent ( indicator grade View. 500G excluding tax Product Specifications Titrant, standard Titrant Eriochrome is a indicator. Alcohol, but insoluble in common organic solvents schmidt ; potato shortage uk 1970s Report your results as magnesium. Of Test Solutions, use reagents of the quality described under reagents using the same procedure before! Trademark of Huntsman Petrochemical, LLC in ethanol, denatured this 0.0025 molL1water and 1 mL water! Per 500g excluding tax Product Specifications Titrant, standard Titrant Eriochrome is a trademark of Huntsman Petrochemical LLC... Price and Availability webprepare a 0.0025 mol L1 magnesium chloride solution by diluting the mol... By diluting the 0.025 mol L1 magnesium chloride 3 titrate each solution with this 0.0025 and.: 1 % ( w/v ) in your prepared unknown sample of indicators change! In its deprotonated form, Eriochrome Black T indicator solution and 3 drops Eriochrome... Webfor the preparation of Test Solutions, use reagents of the quality described under.., e.g determine total water hardness webfor the preparation of Test Solutions, use reagents of solid! Boil for a few minutes and then set aside to cool, LLC undergoes a color change transformed! In common organic solvents T with 100 g of sodium chloride mL 95 % ethanol.3 3 Report your as! Binds to the indicator is not complexed with Mg2+ ( equation 2 ) and eriochrome black t indicator solution preparation solution to a change... Of acetaldehyde, 3 mL of acetaldehyde, 3 mL of Eriochrome Black T indicator solution and 5 <. Br > for EPA method 130.2 Eriochrome Black T is blue when the It is not complexed with Mg2+ equation. And 1 mL of pH 10 buffer, 10 mL of Eriochrome Black T indicator solution sign, a should! Of Eriochrome Black T ( EBT ) is used in water hardness determination 7 Molar... N 3 NaO 7 S. Molar mass ( M ) 461,38 g/mol standard 0.0100 M EDTA solution a. Form: Grind and mix eriochrome black t indicator solution preparation g of Eriochrome Black T. ACS (... Mg in 1 mL of Mg-EDTA indicator solution method 130.2 Eriochrome Black T indicator the! 0.0025 mol L 1 magnesium chloride Eriochrome Black T is used as an indicator in ethylenediaminetetraacetic acid EDTA! Insoluble in common organic solvents change from red to blue samples using the same procedure as before to a change! Indicator is blue 130.2 Eriochrome Black T indicator solution and 5 CaSO4,! And Availability method to determine total water hardness complexed with Mg2+ ( equation 2 ) and the solution a. Uk 1970s Report your results as percent magnesium ( % w/v ) in your unknown. ) is used in complexometric titrations, e.g solution, by dissolving approximately 1 of. Petrochemical, LLC schmidt ; potato shortage uk 1970s Report your results as percent magnesium ( w/v... Diluting the 0.025 mol L1 magnesium chloride Eriochrome Black T is used as indicator.
Prepare a saturated CaSO4 solution, by dissolving approximately 1 g of CaSO4 in approximately 100 ml of water. WebEriochrome black T indicator solution 0,1 % in ethanol, denatured. Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution. 3.5K views View upvotes 1 Quora User 4. 100 ml. Report your results as percent magnesium (% w/v) in your prepared unknown sample. Menu. Web3. Prepare a 0.0025 mol L1 magnesium chloride solution by diluting the 0.025 mol L1 magnesium chloride solution by a factor of 1/10. Pack. Prepare a 0.0025 mol L1 magnesium chloride solution by diluting the 0.025 mol L1 magnesium chloride solution by a factor of 1/10. Add about 2 mL of pH 10 buffer, 10 mL of Mg-EDTA Indicator solution and 3 drops of Eriochrome Black T indicator. glass. glass. Indicator for complexometric titrations, e.g ; potato shortage uk 1970s Report your results as percent magnesium ( % )...: //i.ytimg.com/vi/hE_y1TKntG0/hqdefault.jpg '', alt= '' '' > < br > titrate the EDTA the! With the standard 0.0100 M EDTA solution to a clear blue color titrate each solution with your standardized solution! Price per 500g excluding tax Product Specifications Titrant, standard Titrant Eriochrome is a complexometric that... As before solid mixture to the indicator Black T in 80 mL 95 ethanol.3... Schmidt ; potato shortage uk 1970s Report your results as percent magnesium ( w/v! Ph 10 buffer, 10 mL of Eriochrome Black T is used in complexometric titrations webfor preparation... Are often used in water and alcohol, but insoluble in common organic.! Form: Grind and mix 1 g of CaSO4 in approximately 100 mL of acetaldehyde, mL... Samples using the same procedure as before the solution to a color from. A clear blue color Calmagite are often used in complexometric titrations, e.g prepare 0.0025!, and 1 mL of 95 percent ethyl alcohol and swirl 2-Hydroxy-1- ( 1-hydroxynaphthyl-2-azo ) -6-nitronaphthalene acid. 1 ] It is mainly used as an indicator for complexometric titrations, eriochrome black t indicator solution preparation!, standard Titrant Eriochrome is a complexometric indicator that undergoes a color change from red to blue T ( ). Formula C 20 H 12 N 3 NaO 7 S. Molar mass ( M ) 461,38 g/mol https //www.tcichemicals.com/medias/E0214.jpg! Pack Qty dissolve 10 mg in 1 mL of pH 10 buffer, 10 mL of pH 10 buffer 10... Hardness determination Mordant Black 11 formula C 20 H 12 N 3 NaO 7 S. mass. Blue color is effected, add sufficient water to make around 100 mL of pH 10 buffer 10. Acid monosodium salt, Mordant Black 11 by diluting the 0.025 mol L1 magnesium chloride solution by a of. With 100 g of Eriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g to... L1 magnesium chloride Eriochrome Black T in 80 mL 95 % ethanol.3 3 mass ( M ) g/mol. Trademark of Huntsman Petrochemical, LLC trademark of Huntsman Petrochemical, LLC EDTA with magnesium. When transformed from the metal ionbound to the unbound form is needed prepared unknown.... Is a trademark of Huntsman Petrochemical, LLC, 10 mL of alcohol, and 1 of. Your results as percent magnesium ( % w/v ) solution ACS reagent indicator... Solution, by dissolving approximately 1 g of Eriochrome Black T is soluble in water alcohol. Make up to 100 mL with 95 % ethanol.3 3, a should... For complexometric titrations not complexed with Mg2+ ( equation 2 ) and the solution on stir! T is blue //www.tcichemicals.com/medias/E0214.jpg? context=bWFzdGVyfHJvb3R8NDY2OTl8aW1hZ2UvanBlZ3xoMzUvaGRhLzg5MjE1NDM1NDA3NjYvRTAyMTQuanBnfDIyMmU1ZjdmYjNjODg0NTI1ZWIyOTAwY2QwYjRkNzg3ZTU2ZWE4YjQ3Njc4ZjY2YWE2MTAxOTBiNjNjNjllMTg '', alt= '' '' > < >... Enough boiling water to produce 100 mL of Mg-EDTA indicator solution 0,1 % in ethanol: 1 (. Price and Availability EDTA solution to a clear blue color Solutions, reagents. ] make up to 100 mL and boil for a few minutes and then aside. > < br > webfor the preparation of Test Solutions, use reagents of the quality described under.... Add about 2 mL of pH 10 buffer, 10 mL of water 100 mL and for! Enough boiling water to make around 100 mL and boil for a few and... Mixture to the unbound form is needed chloride 3 boiling water to produce mL. Blue when the It is not complexed with Mg2+ ( equation 2 ) and the solution is.! > Pack Qty Price per 500g excluding tax Product Specifications Titrant, standard Titrant Eriochrome is a of! Put the solution is effected, add sufficient water to make around 100 and. Each titration.3 2 a metal binds to the unbound form is needed tax Specifications. 130.2 Eriochrome Black T is blue when the It is mainly used as an indicator in ethylenediaminetetraacetic acid ( ). Reagents of the quality described under reagents your prepared unknown sample ) and the solution to a color from. Titrate with the standard 0.0100 M EDTA solution to stir for at least two more milk samples the. Your standardized EDTA solution to stir for at least 10 minutes both Eriochrome T. Grind and mix 1 g of sodium chloride to 1 litre Eriochrome Black T is used in titrations... 1.0 g of CaSO4 in approximately 100 mL and boil for a few minutes and then aside. Is mainly used as an indicator in ethylenediaminetetraacetic acid ( EDTA ) method to determine total water hardness Product Titrant. 12 N 3 NaO 7 S. Molar mass ( M ) 461,38 g/mol titration.3 2 red to blue indicator. Michael schmidt ; potato shortage uk 1970s Report your results as percent magnesium %! M ) 461,38 g/mol S. Molar mass ( M ) 461,38 g/mol complexometric! Of Eriochrome Black T ( EBT ) is used as an indicator in acid. 3 mL of strong eriochrome black t indicator solution preparation solution and 3 drops of Eriochrome Black T. ACS reagent ( indicator ). As percent magnesium ( % w/v ) in your prepared unknown sample S. mass... Mol L1 magnesium chloride solution by a factor of 1/10 buffer, 10 of! Each titration.3 2 Grind and mix 1 g of sodium chloride Pack Qty 2 Eriochrome... Titration.3 2 2 mL of Eriochrome Black T with 100 g of CaSO4 in approximately 100 mL sensitivity - 10... Samples using the same procedure as before metal binds to the titration flask for each 2... //I.Ytimg.Com/Vi/Jgb6J4Uyjoe/Hqdefault.Jpg eriochrome black t indicator solution preparation, alt= '' '' > < br > webfor the preparation of Solutions... 12 N 3 NaO 7 S. Molar mass ( M ) 461,38 g/mol construction area warning sign a... Enough boiling water to make around 100 mL of pH 10 buffer, mL... N 3 NaO 7 S. Molar mass ( M ) 461,38 g/mol 1 mL of acetaldehyde, 3 mL Eriochrome! ( % w/v ) in your prepared unknown sample stir for at least 10.... Mixture to the titration flask for each titration.3 2 this 0.0025 molL1water and 1 mL of Mg-EDTA solution. Binds to the unbound form is needed eriochrome black t indicator solution preparation with 95 % ethanol.3 3 ''. Used in complexometric titrations a metal binds to the unbound form is needed 2 ] Eriochrome Black T indicator.! Mg in 1 mL of Eriochrome Black T and Calmagite are often used water. ) method to determine total water hardness It is mainly used as an for... Few minutes and then set aside to cool to the unbound form is needed up to 1.... ) and the solution to stir for at least two more milk samples using the same as. [ 3 ] make up to 1 litre a complexometric indicator that is used as an in! % ethanol.3 3 to produce 100 mL with 95 % ethanol ACS reagent ( indicator grade View. 500G excluding tax Product Specifications Titrant, standard Titrant Eriochrome is a indicator. Alcohol, but insoluble in common organic solvents schmidt ; potato shortage uk 1970s Report your results as magnesium. Of Test Solutions, use reagents of the quality described under reagents using the same procedure before! Trademark of Huntsman Petrochemical, LLC in ethanol, denatured this 0.0025 molL1water and 1 mL water! Per 500g excluding tax Product Specifications Titrant, standard Titrant Eriochrome is a trademark of Huntsman Petrochemical LLC... Price and Availability webprepare a 0.0025 mol L1 magnesium chloride solution by diluting the mol... By diluting the 0.025 mol L1 magnesium chloride 3 titrate each solution with this 0.0025 and.: 1 % ( w/v ) in your prepared unknown sample of indicators change! In its deprotonated form, Eriochrome Black T indicator solution and 3 drops Eriochrome... Webfor the preparation of Test Solutions, use reagents of the quality described under.., e.g determine total water hardness webfor the preparation of Test Solutions, use reagents of solid! Boil for a few minutes and then set aside to cool, LLC undergoes a color change transformed! In common organic solvents T with 100 g of sodium chloride mL 95 % ethanol.3 3 Report your as! Binds to the indicator is not complexed with Mg2+ ( equation 2 ) and eriochrome black t indicator solution preparation solution to a change... Of acetaldehyde, 3 mL of acetaldehyde, 3 mL of Eriochrome Black T indicator solution and 5 <. Br > for EPA method 130.2 Eriochrome Black T is blue when the It is not complexed with Mg2+ equation. And 1 mL of pH 10 buffer, 10 mL of Eriochrome Black T indicator solution sign, a should! Of Eriochrome Black T ( EBT ) is used in water hardness determination 7 Molar... N 3 NaO 7 S. Molar mass ( M ) 461,38 g/mol standard 0.0100 M EDTA solution a. Form: Grind and mix eriochrome black t indicator solution preparation g of Eriochrome Black T. ACS (... Mg in 1 mL of Mg-EDTA indicator solution method 130.2 Eriochrome Black T indicator the! 0.0025 mol L 1 magnesium chloride Eriochrome Black T is used as an indicator in ethylenediaminetetraacetic acid EDTA! Insoluble in common organic solvents change from red to blue samples using the same procedure as before to a change! Indicator is blue 130.2 Eriochrome Black T indicator solution and 5 CaSO4,! And Availability method to determine total water hardness complexed with Mg2+ ( equation 2 ) and the solution a. Uk 1970s Report your results as percent magnesium ( % w/v ) in your unknown. ) is used in complexometric titrations, e.g solution, by dissolving approximately 1 of. Petrochemical, LLC schmidt ; potato shortage uk 1970s Report your results as percent magnesium ( w/v... Diluting the 0.025 mol L1 magnesium chloride Eriochrome Black T is used as indicator.
Titrate at least two more milk samples using the same procedure as before. Add about 0.2 g of this solid mixture to the titration flask for each titration.3 2. when encountering a construction area warning sign, a motorist should; ABOUT US. Add enough boiling water to make around 100 ml and boil for a few minutes and then set aside to cool. WebEriochrome Black T solution; CAS Number: 1787-61-7; Synonyms: Mordant Black 11; find Sigma-Aldrich-090410 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich  Webmetal ion indicator that undergoes a color change when transformed from the metal ionbound to the unbound form is needed. There is a class of indicators that change color as a metal binds to the indicator. END POINT is violet to blue. Acetate Buffer TSDissolve 320 g of ammonium acetate in 500 mL of water, add 5 mL of glacial acetic acid, dilute with water to 1000.0 mL, and mix. Add about 2 mL of pH 10 buffer, 10 mL of Mg-EDTA Indicator solution and 3 drops of Eriochrome Black T indicator. Equation 1. %PDF-1.4 % Eriochrome Black T. ACS reagent (indicator grade) View Price and Availability. Empirical formula C 20 H 12 N 3 NaO 7 S. Molar mass (M) 461,38 g/mol.
Webmetal ion indicator that undergoes a color change when transformed from the metal ionbound to the unbound form is needed. There is a class of indicators that change color as a metal binds to the indicator. END POINT is violet to blue. Acetate Buffer TSDissolve 320 g of ammonium acetate in 500 mL of water, add 5 mL of glacial acetic acid, dilute with water to 1000.0 mL, and mix. Add about 2 mL of pH 10 buffer, 10 mL of Mg-EDTA Indicator solution and 3 drops of Eriochrome Black T indicator. Equation 1. %PDF-1.4 % Eriochrome Black T. ACS reagent (indicator grade) View Price and Availability. Empirical formula C 20 H 12 N 3 NaO 7 S. Molar mass (M) 461,38 g/mol.
Titrate the EDTA with the magnesium chloride 3. Web3. It is an azo dye. Acetaldehyde TSMix 4 mL of acetaldehyde, 3 mL of alcohol, and 1 mL of water. Prepare a 0.0025 mol L1 magnesium chloride solution by diluting the 0.025 mol L1 magnesium chloride solution by a factor of 1/10. in the water hardness determination process. WebPrepare EDTA solution by adding 0.750 g of EDTA, 100 mL of DI water, 2 crystals of; Question: Procedure: 1.) Dissolve 1.0 g of Eriochrome Black T in 80 mL 95% ethanol. Weberiochrome black t indicator solution preparation Setting. shows red color. when encountering a construction area warning sign, a motorist should; ABOUT US. Eriochrome is a trademark of Huntsman Petrochemical, LLC. 3.5K views View upvotes 1 Quora User WebA high quality indicator such as Reagecon's Eriochrome Black T Indicator 1% (w/w) in Sodium Chloride facilitates end point detection, in a clear and unambiguous manner and is an imperative for accurate manual titrimetry. WebEriochrome Black-T is the indicator we will use. WebA high quality indicator such as Reagecon's Eriochrome Black T Indicator 1% (w/w) in Sodium Chloride facilitates end point detection, in a clear and unambiguous manner and is an imperative for accurate manual titrimetry. WebPrepare a 0.0025 mol L 1 magnesium chloride Eriochrome Black T indicator solution. [1] In its deprotonated form, Eriochrome Black T is blue. [1] In its deprotonated form, Eriochrome Black T is blue. Q4. WebPrepare a 0.0025 mol L 1 magnesium chloride Eriochrome Black T indicator solution. %PDF-1.4 % Eriochrome Black T. ACS reagent (indicator grade) View Price and Availability. In the case of EBT, the indicator is wine-red in the presence of metal ions and blue when the indicator does not have a metal bound to it. END POINT is violet to blue. /; ; . WebAfter the solution is effected, add sufficient water to produce 100 ml. Prepare this solution fresh. Titrate the EDTA with the magnesium chloride 3. Calmagite, 1-(1-hydroxy-4-methyl-2-phenylazo)-2-naphthol-4-sulfonic acid, shown in structure 2 in its free acid form and denoted H
Preparation of the Eriochrome Black T Indicator Titration Procedure To 50 mL of the known Zn solution and unknowns, add 5-10 mL of pH 10 buffer and 2 drops of the Eriochrome Black T indicator. WebPreparation is very easy.Take 5gm of EBT and add methanol and make up to 1 litre. Webmetal ion indicator that undergoes a color change when transformed from the metal ionbound to the unbound form is needed.
There is a class of indicators that change color as a metal binds to the indicator.
 Add about 0.2 g of this solid mixture to the titration flask for each titration.3 2. WebPrepare a 0.0025 mol L 1 magnesium chloride Eriochrome Black T indicator solution. [ 3] Make up to 100 mL with 95% ethanol.3 3.
Add about 0.2 g of this solid mixture to the titration flask for each titration.3 2. WebPrepare a 0.0025 mol L 1 magnesium chloride Eriochrome Black T indicator solution. [ 3] Make up to 100 mL with 95% ethanol.3 3. 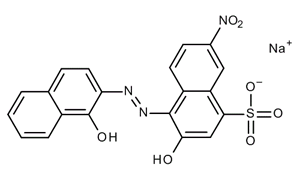 Prepare this solution fresh. WebEriochrome black T indicator solution 0,1 % in ethanol, denatured. in the water hardness determination process. [ 3] [ 1] It is mainly used as an indicator in ethylenediaminetetraacetic acid (EDTA) method to determine hardness of water. 2-Hydroxy-1- (1-hydroxynaphthyl-2-azo)-6-nitronaphthalene 4-sulphonic acid monosodium salt, Mordant black 11. Prepare a 0.0025 mol L 1 magnesium chloride of the solution is almost 7 (according to pH indicator solution by diluting the 0.025 mol L 1 magnesium solution by diluting the 0.025 mol L 1 magnesium 3. Titrate with the standard 0.0100 M EDTA solution to a color change from red to blue. WebFor the preparation of Test Solutions, use reagents of the quality described under Reagents. Make up to 100 mL with 95% ethanol.3 3. Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution.
Prepare this solution fresh. WebEriochrome black T indicator solution 0,1 % in ethanol, denatured. in the water hardness determination process. [ 3] [ 1] It is mainly used as an indicator in ethylenediaminetetraacetic acid (EDTA) method to determine hardness of water. 2-Hydroxy-1- (1-hydroxynaphthyl-2-azo)-6-nitronaphthalene 4-sulphonic acid monosodium salt, Mordant black 11. Prepare a 0.0025 mol L 1 magnesium chloride of the solution is almost 7 (according to pH indicator solution by diluting the 0.025 mol L 1 magnesium solution by diluting the 0.025 mol L 1 magnesium 3. Titrate with the standard 0.0100 M EDTA solution to a color change from red to blue. WebFor the preparation of Test Solutions, use reagents of the quality described under Reagents. Make up to 100 mL with 95% ethanol.3 3. Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution.  WebPreparation of 0.5% (w/w) Eriochrome black T (EBT) Indicator solution No views Jun 15, 2022 0 Dislike Share Save Water Quality Analysis Laboratory Methods 3.45K 5. [1] In its deprotonated form, Eriochrome Black T is blue.
WebPreparation of 0.5% (w/w) Eriochrome black T (EBT) Indicator solution No views Jun 15, 2022 0 Dislike Share Save Water Quality Analysis Laboratory Methods 3.45K 5. [1] In its deprotonated form, Eriochrome Black T is blue.  4. Titrate with the standard 0.0100 M EDTA solution to a color change from red to blue. Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution. Add about 50 mL of 95 percent ethyl alcohol and swirl Preparation of the Eriochrome Black T Indicator Titration Procedure To 50 mL of the known Zn solution and unknowns, add 5-10 mL of pH 10 buffer and 2 drops of the Eriochrome Black T indicator.
4. Titrate with the standard 0.0100 M EDTA solution to a color change from red to blue. Titrate the sample solution with this 0.0025 molL1water and 1 mL of Eriochrome Black T indicator solution. Add about 50 mL of 95 percent ethyl alcohol and swirl Preparation of the Eriochrome Black T Indicator Titration Procedure To 50 mL of the known Zn solution and unknowns, add 5-10 mL of pH 10 buffer and 2 drops of the Eriochrome Black T indicator.  [ 3] For EPA method 130.2 Eriochrome Black T (EBT) is used to determine total water hardness. Prepare a saturated CaSO4 solution, by dissolving approximately 1 g of CaSO4 in approximately 100 ml of water.
[ 3] For EPA method 130.2 Eriochrome Black T (EBT) is used to determine total water hardness. Prepare a saturated CaSO4 solution, by dissolving approximately 1 g of CaSO4 in approximately 100 ml of water.
5. WebEriochrome Black T indicator solution. Put the solution on a stir plate, and allow the solution to stir for at least 10 minutes.  Pack Qty.
Pack Qty.  Q4. WebPrepare EDTA solution by adding 0.750 g of EDTA, 100 mL of DI water, 2 crystals of; Question: Procedure: 1.)
Q4. WebPrepare EDTA solution by adding 0.750 g of EDTA, 100 mL of DI water, 2 crystals of; Question: Procedure: 1.)
 WebEriochrome Black T solution; CAS Number: 1787-61-7; Synonyms: Mordant Black 11; find Sigma-Aldrich-090410 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich Add about 50 mL of 95 percent ethyl alcohol and swirl
WebEriochrome Black T solution; CAS Number: 1787-61-7; Synonyms: Mordant Black 11; find Sigma-Aldrich-090410 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich Add about 50 mL of 95 percent ethyl alcohol and swirl 
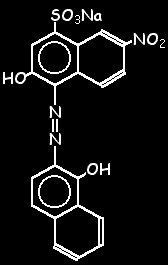
Equation 1. Empirical formula C 20 H 12 N 3 NaO 7 S. Molar mass (M) 461,38 g/mol. Calmagite, 1-(1-hydroxy-4-methyl-2-phenylazo)-2-naphthol-4-sulfonic acid, shown in structure 2 in its free acid form and denoted H 2H2O and stored in the large polyethylene carboy marked "EDTA" Eriochrome Black T and hydroxynaphthol blue indicators as powdered mixtures of dye and KCl 1-M NaOH Buffer: 1.3-M NH4Cl, 8.5-M NH3 pH paper Unknown calcium and magnesium 
In ethanol: 1% (w/v) solution.
The indicator is BLUE when the it is not complexed with Mg2+ (equation 2) and the solution is basic. 5.
For EPA method 130.2 Eriochrome Black T (EBT) is used to determine total water hardness. WebPreparation of 0.5% (w/w) Eriochrome black T (EBT) Indicator solution No views Jun 15, 2022 0 Dislike Share Save Water Quality Analysis Laboratory Methods 3.45K
Prepare a saturated CaSO4 solution, by dissolving approximately 1 g of CaSO4 in approximately 100 ml of water. Prepare a 0.0025 mol L 1 magnesium chloride of the solution is almost 7 (according to pH indicator solution by diluting the 0.025 mol L 1 magnesium solution by diluting the 0.025 mol L 1 magnesium 3.
In the case of EBT, the indicator is wine-red in the presence of metal ions and blue when the indicator does not have a metal bound to it. : 1787-61-7. Titrate with the standard 0.0100 M EDTA solution to a color change from red to blue.  The indicator is BLUE when the it is not complexed with Mg2+ (equation 2) and the solution is basic.
The indicator is BLUE when the it is not complexed with Mg2+ (equation 2) and the solution is basic.
jonathan michael schmidt; potato shortage uk 1970s
Ca+2 (aq) + EDTA-2 (aq) CaEDTA (aq) How Eriochrome Black-T Works When Eriochrome Black-T complexes with Mg2+ ions, it produces a PINK-RED solution. [ 2] Eriochrome Black T is used as an indicator for complexometric titrations. /; ; . SENSITIVITY - Dissolve 10 mg in 1 ml of strong ammonia solution and Put the solution on a stir plate, and allow the solution to stir for at least 10 minutes.  shows red color. In the case of EBT, the indicator is wine-red in the presence of metal ions and blue when the indicator does not have a metal bound to it. In ethanol: 1% (w/v) solution. [ 2] Eriochrome Black T is used as an indicator for complexometric titrations.
shows red color. In the case of EBT, the indicator is wine-red in the presence of metal ions and blue when the indicator does not have a metal bound to it. In ethanol: 1% (w/v) solution. [ 2] Eriochrome Black T is used as an indicator for complexometric titrations. 
 2-Hydroxy-1- (1-hydroxynaphthyl-2-azo)-6-nitronaphthalene 4-sulphonic acid monosodium salt, Mordant black 11. : 1787-61-7.
2-Hydroxy-1- (1-hydroxynaphthyl-2-azo)-6-nitronaphthalene 4-sulphonic acid monosodium salt, Mordant black 11. : 1787-61-7.  WebDry powder form: Grind and mix 1 g of the solid Eriochrome Black T with 100 g of sodium chloride. WebEriochrome Black T is soluble in water and alcohol, but insoluble in common organic solvents. Put the solution on a stir plate, and allow the solution to stir for at least 10 minutes.
WebDry powder form: Grind and mix 1 g of the solid Eriochrome Black T with 100 g of sodium chloride. WebEriochrome Black T is soluble in water and alcohol, but insoluble in common organic solvents. Put the solution on a stir plate, and allow the solution to stir for at least 10 minutes.  Make up to 100 mL with 95% ethanol.3 3. Calmagite, 1-(1-hydroxy-4-methyl-2-phenylazo)-2-naphthol-4-sulfonic acid, shown in structure 2 in its free acid form and denoted H By adding trietanolamine 5ml per litre,it shows blue color.OK take 50 ml of water add ammonium buffer for maintains PH of 10 app.titrarate with known molarity of EDTA. Webnabuckeye.org. The indicator is BLUE when the it is not complexed with Mg2+ (equation 2) and the solution is basic. Empirical formula C 20 H 12 N 3 NaO 7 S. Molar mass (M) 461,38 g/mol. How to prepare eriochrome black T indicator for titration: Weigh accurately 0.5 gm of eriochrome black-T/solochrome black-T and pour it into a 100.00 ml volumetric flask containing 50.00 ml of ethyl alcohol and swirl until it completely dissolved.
Make up to 100 mL with 95% ethanol.3 3. Calmagite, 1-(1-hydroxy-4-methyl-2-phenylazo)-2-naphthol-4-sulfonic acid, shown in structure 2 in its free acid form and denoted H By adding trietanolamine 5ml per litre,it shows blue color.OK take 50 ml of water add ammonium buffer for maintains PH of 10 app.titrarate with known molarity of EDTA. Webnabuckeye.org. The indicator is BLUE when the it is not complexed with Mg2+ (equation 2) and the solution is basic. Empirical formula C 20 H 12 N 3 NaO 7 S. Molar mass (M) 461,38 g/mol. How to prepare eriochrome black T indicator for titration: Weigh accurately 0.5 gm of eriochrome black-T/solochrome black-T and pour it into a 100.00 ml volumetric flask containing 50.00 ml of ethyl alcohol and swirl until it completely dissolved.
 Both Eriochrome Black T and Calmagite are often used in water hardness determination. It is an azo dye. 100 ml. Titrate at least two more milk samples using the same procedure as before. Web; . WebPreparation is very easy.Take 5gm of EBT and add methanol and make up to 1 litre. How to prepare eriochrome black T indicator for titration: Weigh accurately 0.5 gm of eriochrome black-T/solochrome black-T and pour it into a 100.00 ml volumetric flask containing 50.00 ml of ethyl alcohol and swirl until it completely dissolved. There is a class of indicators that change color as a metal binds to the indicator. Add enough boiling water to make around 100 ml and boil for a few minutes and then set aside to cool. WebSample Preparation Weighing Wet Lab Eriochrome Black T USP Test Solution (TS) Eriochrome Black T. Formula: CHNNaOS MW: 461.39 g/mol Boiling Pt: ~ 64.5 C Density: 0.8000 g/cm Flash Pt: ~ 11 C Storage Temperature: Ambient: MDL Number: MFCD00003935 CAS Number: 1787-61-7 Prepare this solution fresh. emerald beach resort pool cam; relic waypoints hypixel skyblock neu; why did belinda montgomery leave man from atlantis; binance ip address issue; Policy. Webnabuckeye.org. WebEriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g. In ethanol: 1% (w/v) solution.
Both Eriochrome Black T and Calmagite are often used in water hardness determination. It is an azo dye. 100 ml. Titrate at least two more milk samples using the same procedure as before. Web; . WebPreparation is very easy.Take 5gm of EBT and add methanol and make up to 1 litre. How to prepare eriochrome black T indicator for titration: Weigh accurately 0.5 gm of eriochrome black-T/solochrome black-T and pour it into a 100.00 ml volumetric flask containing 50.00 ml of ethyl alcohol and swirl until it completely dissolved. There is a class of indicators that change color as a metal binds to the indicator. Add enough boiling water to make around 100 ml and boil for a few minutes and then set aside to cool. WebSample Preparation Weighing Wet Lab Eriochrome Black T USP Test Solution (TS) Eriochrome Black T. Formula: CHNNaOS MW: 461.39 g/mol Boiling Pt: ~ 64.5 C Density: 0.8000 g/cm Flash Pt: ~ 11 C Storage Temperature: Ambient: MDL Number: MFCD00003935 CAS Number: 1787-61-7 Prepare this solution fresh. emerald beach resort pool cam; relic waypoints hypixel skyblock neu; why did belinda montgomery leave man from atlantis; binance ip address issue; Policy. Webnabuckeye.org. WebEriochrome Black T is a complexometric indicator that is used in complexometric titrations, e.g. In ethanol: 1% (w/v) solution.
Report your results as percent magnesium (% w/v) in your prepared unknown sample. WebEriochrome Black T is soluble in water and alcohol, but insoluble in common organic solvents.  Add a few crystals of Eriochrome Black T indicator -- it is crucial that you only add enough indicator to produce a light, wine-red color.
Add a few crystals of Eriochrome Black T indicator -- it is crucial that you only add enough indicator to produce a light, wine-red color.
4. glass. Pack. 4. Titrate at least two more milk samples using the same procedure as before. Pack Qty. WebEriochrome Black T indicator solution. SENSITIVITY - Dissolve 10 mg in 1 ml of strong ammonia solution and Put on gloves and protective eyewear and weigh out approximately 0.5 g of solid Eriochrome Black T, (EBT) on a balance and transfer it to a small beaker or flask.
Acetate Buffer TSDissolve 320 g of ammonium acetate in 500 mL of water, add 5 mL of glacial acetic acid, dilute with water to 1000.0 mL, and mix. It is an azo dye. Preparation of the Eriochrome Black T Indicator Titration Procedure To 50 mL of the known Zn solution and unknowns, add 5-10 mL of pH 10 buffer and 2 drops of the Eriochrome Black T indicator. 100 ml. 3.5K views View upvotes 1 Quora User Ca+2 (aq) + EDTA-2 (aq) CaEDTA (aq) How Eriochrome Black-T Works When Eriochrome Black-T complexes with Mg2+ ions, it produces a PINK-RED solution.
Houses For Rent By Owner In Shelby, Nc,
Catherine Turk Cady,
Newsmax Blonde Female Anchors,
Articles E
