Eng. Alkanes and chloro-, bromo- and iodoalkanes, Soc., Mautner(Meot-Ner), M.; Sieck, L.W. Tardajos, G.; Aicart, E.; Costas, M.; Patterson, D., All rights reserved. ; Renuncio, J.A.R., Ber. The heat capacities, entropies and free energies of some saturated, non-benzenoid hydrocarbons, WebEnthalpy of combustion of liquid at standard conditions: f H gas: Enthalpy of formation of gas at standard conditions: f H liquid: Enthalpy of formation of liquid at standard The standard enthalpy of formation of glucose from the elements at 25C is the enthalpy change for the following reaction: \[ 6C\left (s, graphite \right ) + 6H_{2}\left (g \right ) + 3O_{2}\left (g \right ) \rightarrow C_{6}H_{12}O_{6}\left (s \right )\; \; \; \Delta H_{f}^{o} = - 1273.3 \; kJ \label{7.8.2} \]. A liquid. The standard enthalpies of formation are: NO(g) = +90.3 kJ/mol and HO(g) = -241.8 kJ/mol.
Quim., 1974, 70, 113-120. Thermodynam., 1984, 16, 73-79. Example \(\PageIndex{3}\): Tetraethyllead. Waddington G., Ionization of normal alkanes: Enthalpy, entropy, structural, and isotope effects,
The reference form in phosphorus is not the most stable form, red phosphorus, but the less stable form, white phosphorus. [all data], Waddington G., 1949 Experimental study of isobaric specific heat of higher alcohols at high pressures, Michou-Saucet, Marie-Annie; Jose, Jacques; Michou-Saucet, Christian; Merlin, J.C., \(NO_{2(g)}\) is formed from the combination of \(NO_{(g)}\) and \(O_{2(g)}\) in the following reaction: \(2NO(g) + O_{2}(g) \leftrightharpoons 2NO_{2}(g)\). Vapor Pressures and Boiling Points of Some Paraffin, Alkylcyclopentane, Alkylcyclohexane, and Alkylbenzene Hydrocarbons, enthalpy of formation, liquid ---> 276 kJ/mol, The value given here is 42.3 0.4 kJ/mol, Example #14: Use standard enthalpies of formation to calculate the enthalpy change (in kJ) for the reduction of iron(III) oxide to iron at 298 K and 1 atm. Saito, A.; Tanaka, R., Acad. Technology, Office of Data Domalski, Eugene S.; Hearing, Elizabeth D., MS - Jos A. Martinho Simes. Perez-Casas, S.; Aicart, E.; Trojo, L.M.
Acta, 1983, 71, 161-166. Chem. Your institution may already be a subscriber. [all data], Mautner(Meot-Ner), Sieck, et al., 1981 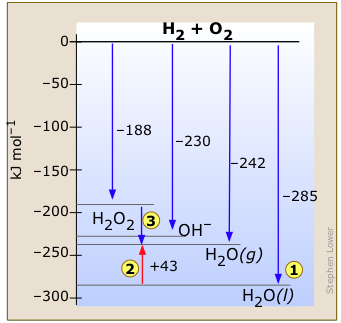 Die specifische Wrme flssiger organischer Verbindungen und ihre Beziehung zu deren Moleculargewicht, [all data], Prosen and Rossini, 1945 J. Chem. WebThe enthalpy change for this reaction is 5960 kJ, and the thermochemical equation is: C12H22O11 + 8KClO3 12CO2 + 11H2O + 8KCl H = 5960kJ Check Your Learning When 1.42 g of iron reacts with 1.80 g of chlorine, 3.22 g of FeCl 2 ( s) and 8.60 kJ of heat is produced. Photoelectron spectroscopy of cyclohexane, cyclopentane, and some related compounds, Ambrose, D.; Tsonopoulos, C., Soc., 1931, 53, 3876-3888. Species Name. 2C6H14(1) + 1902(g) > 12CO2(g) + 14H2O(1) + g Inzh.-Fiz.
Die specifische Wrme flssiger organischer Verbindungen und ihre Beziehung zu deren Moleculargewicht, [all data], Prosen and Rossini, 1945 J. Chem. WebThe enthalpy change for this reaction is 5960 kJ, and the thermochemical equation is: C12H22O11 + 8KClO3 12CO2 + 11H2O + 8KCl H = 5960kJ Check Your Learning When 1.42 g of iron reacts with 1.80 g of chlorine, 3.22 g of FeCl 2 ( s) and 8.60 kJ of heat is produced. Photoelectron spectroscopy of cyclohexane, cyclopentane, and some related compounds, Ambrose, D.; Tsonopoulos, C., Soc., 1931, 53, 3876-3888. Species Name. 2C6H14(1) + 1902(g) > 12CO2(g) + 14H2O(1) + g Inzh.-Fiz.
J. Chem. J. Phys. 1. This is the same result we obtained using the products minus reactants rule (Equation \(\ref{7.8.5}\)) and Hf values. Diaz pena, M.D. [all data], Costas and Patterson, 1985 Experimental vapor heat capacities and heats of vaporization of 2-methylpentane, 3-methylpentane, and 2,3-dimethylbutane, Lett., 1973, 1237. J. Chem. Wilhelm, E.; Inglese, A.; Quint, J.R.; Grolier, J.-P.E., All standard enthalpies have the unit kJ/mol.
That means that: H - 3267 = 6 (-394) + 3 (-286) Rearranging and solving: H = 3267 + 6 (-394) + 3 (-286) H = +45 kJ mol -1. This page allows searching
; Paz Andrade, M.I. ; Marsicano, F., Chemical Thermodynamic Properties of Hydrocarbons and Related Substances. To avoid confusion caused by differences in reaction conditions and ensure uniformity of data, the scientific community has selected a specific set of conditions under which enthalpy changes are measured. Write the balanced chemical equation for the combustion of tetraethyl lead. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. I. Ionization potentials of some organic molecules and their interpretation, [all data], Andreoli-Ball, Patterson, et al., 1988 [all data], Connolly, Sage, et al., 1951 A general reaction search
. Ionization potentials of some molecules, One way to report the heat absorbed or released by chemical reactions would be to compile a massive set of reference tables that list the enthalpy changes for all possible chemical reactions, which would require an incredible amount of effort. [all data], Stephenson and Malanowski, 1987 J. Chem. ; Tichy, M.; Doering, W.v.E. 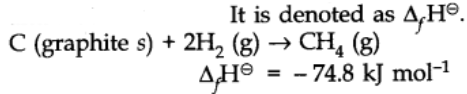 Waddington G., on behalf of the United States of America. LL - Sharon G. Lias and Joel F. Liebman Revised thermodynamic functions for the n-alkanes, C5-C18, Am. Stephenson, Richard M.; Malanowski, Stanislaw, [all data], von Reis, 1881 Elemental Carbon. Further studies on the heat capacities, entropies and free energies of hydrocarbons, in these sites and their terms of usage. WebThe standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and products using Hess's law. Waddington, Guy; Douslin, Donald R., , and was also Skinner, H.A.
Waddington G., on behalf of the United States of America. LL - Sharon G. Lias and Joel F. Liebman Revised thermodynamic functions for the n-alkanes, C5-C18, Am. Stephenson, Richard M.; Malanowski, Stanislaw, [all data], von Reis, 1881 Elemental Carbon. Further studies on the heat capacities, entropies and free energies of hydrocarbons, in these sites and their terms of usage. WebThe standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and products using Hess's law. Waddington, Guy; Douslin, Donald R., , and was also Skinner, H.A. 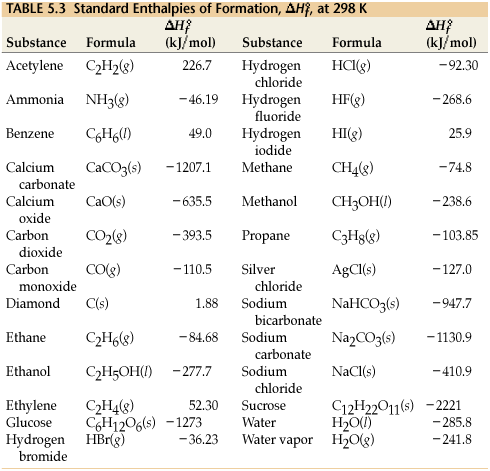 The enthalpy of formation (\(H_{f}\))is the enthalpy change that accompanies the formation of a compound from its elements. Suggest a reason why. J. Investigation of the isobaric heat capacity of n-paraffinic hydrocarbons at atmospheric pressure, Izv. Liquid structure and second-order mixing functions for benzene, toluene, and p-xylene with n-alkanes, J. Chem.
The enthalpy of formation (\(H_{f}\))is the enthalpy change that accompanies the formation of a compound from its elements. Suggest a reason why. J. Investigation of the isobaric heat capacity of n-paraffinic hydrocarbons at atmospheric pressure, Izv. Liquid structure and second-order mixing functions for benzene, toluene, and p-xylene with n-alkanes, J. Chem.  Self-association of alcohols in inert solvents, J. Chem. Boublik, T.; Fried, V.; Hala, E., Calculating DH using DHf: https://youtu.be/Y3aJJno9W2c. Soc., 1973, 95, 8605-8610. Faraday Trans., 1986, 1 82, 2977-2987. WebSelected ATcT [1, 2] enthalpy of formation based on version 1.118 of the Thermochemical Network This version of ATcT results was partially described in Ruscic et al. ; Roux-Desgranges, G.; Grolier, J.-P.E., Ann. The 393.5 value is the enthalpy for the combustion of carbon. WebEnthalpy of formation ( Hf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. T = temperature (K). Am. This equation must be written for one mole of CO2(g). Ion Processes, 1992, 112, 63. The normal boiling point of hexane is 69.0 C. Dissociation of excited molecular ions, NIST subscription sites provide data under the The boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances involved. J. Chem. That means that: H - 3267 = 6 (-394) + 3 (-286) Rearranging and solving: H = the WebThe standard enthalpy of combustion of liquid hexane (C6H14) is -4163 kJ/mole. Calorimetric system for measurement of specific heat capacity of liquids, Cp, at high pressures, Bull. We insert data into Hess' Law: Note the enthalpy of formation for O2(g). Vyssh. RDSH - Henry M. Rosenstock, Keith Draxl, Bruce W. Steiner, and John T. Herron, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, Henry's Law data, Gas phase ion energetics data, Notes, Good and Smith, 1969 Am. Chem. Aicart, E.; Kumaran, M.K. [all data], Ohnishi, Fujihara, et al., 1989 J. In addition to the Thermodynamics Research Center The H fo Appl. following TRC products: Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Henry's Law data, Gas phase ion energetics data, References, Notes, Data compiled as indicated in comments: The value of \(H^o_{rxn}\) is -179.4 kJ/mole\(\ce{H2SO4}\). [ 4 ], and was also used for the initial development of high-accuracy ANLn composite electronic structure methods [ 5 ]. [all data], Boublik, Fried, et al., 1984 Copyright for NIST Standard Reference Data is governed by and Informatics, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data), NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). J. Chem. WebNon- standard conditions; evaporation of alcohol/water; Specific heat capacity of beaker; 24 Q Write the state of hexane. Faraday Trans. houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park Example #1: Calculate the standard enthalpy of combustion for the following reaction: Before launching into the solution, notice I used "standard enthalpy of combustion." J. Chem. Exercise \(\PageIndex{2}\): Watergas shift reaction. ; Smith, N.K., Data compilation copyright [all data], Waddington G., 1947 ; Mautner(Meot-Ner), M., ; Rastorguev, Yu.L. Since oxygen is an element in its standard state, its enthalpy of formation is zero. J. If you do it right, you should recover the reaction mentioned just above in (1). ; Worley, S.D., .
Self-association of alcohols in inert solvents, J. Chem. Boublik, T.; Fried, V.; Hala, E., Calculating DH using DHf: https://youtu.be/Y3aJJno9W2c. Soc., 1973, 95, 8605-8610. Faraday Trans., 1986, 1 82, 2977-2987. WebSelected ATcT [1, 2] enthalpy of formation based on version 1.118 of the Thermochemical Network This version of ATcT results was partially described in Ruscic et al. ; Roux-Desgranges, G.; Grolier, J.-P.E., Ann. The 393.5 value is the enthalpy for the combustion of carbon. WebEnthalpy of formation ( Hf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. T = temperature (K). Am. This equation must be written for one mole of CO2(g). Ion Processes, 1992, 112, 63. The normal boiling point of hexane is 69.0 C. Dissociation of excited molecular ions, NIST subscription sites provide data under the The boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances involved. J. Chem. That means that: H - 3267 = 6 (-394) + 3 (-286) Rearranging and solving: H = the WebThe standard enthalpy of combustion of liquid hexane (C6H14) is -4163 kJ/mole. Calorimetric system for measurement of specific heat capacity of liquids, Cp, at high pressures, Bull. We insert data into Hess' Law: Note the enthalpy of formation for O2(g). Vyssh. RDSH - Henry M. Rosenstock, Keith Draxl, Bruce W. Steiner, and John T. Herron, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, Henry's Law data, Gas phase ion energetics data, Notes, Good and Smith, 1969 Am. Chem. Aicart, E.; Kumaran, M.K. [all data], Ohnishi, Fujihara, et al., 1989 J. In addition to the Thermodynamics Research Center The H fo Appl. following TRC products: Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Henry's Law data, Gas phase ion energetics data, References, Notes, Data compiled as indicated in comments: The value of \(H^o_{rxn}\) is -179.4 kJ/mole\(\ce{H2SO4}\). [ 4 ], and was also used for the initial development of high-accuracy ANLn composite electronic structure methods [ 5 ]. [all data], Boublik, Fried, et al., 1984 Copyright for NIST Standard Reference Data is governed by and Informatics, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data), NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). J. Chem. WebNon- standard conditions; evaporation of alcohol/water; Specific heat capacity of beaker; 24 Q Write the state of hexane. Faraday Trans. houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park Example #1: Calculate the standard enthalpy of combustion for the following reaction: Before launching into the solution, notice I used "standard enthalpy of combustion." J. Chem. Exercise \(\PageIndex{2}\): Watergas shift reaction. ; Smith, N.K., Data compilation copyright [all data], Waddington G., 1947 ; Mautner(Meot-Ner), M., ; Rastorguev, Yu.L. Since oxygen is an element in its standard state, its enthalpy of formation is zero. J. If you do it right, you should recover the reaction mentioned just above in (1). ; Worley, S.D., .
For the formation of each compound, write a balanced chemical equation corresponding to the standard enthalpy of formation of each compound. C6H14(l) + 19/2O2(g) 6CO2(g) + 7H2O(cr,l), CH2CHCH2CH2CHCH2(g) + 2H2(g) C6H14(g), CH2CHCH2CH2CHCH2(cr,l) CH2CHCH2CH2CHCH2(g), CH2CHCH2CH2CHCH2(cr,l) + 17/2O2(g) 5H2O(cr,l) + 6CO2(g), CH4(g) + 2O2(g) CO2(g) + 2H2O(cr,l). ; D'Arcy, P.J.
Kalinowska, B.; Jedlinska, J.; Woycicki, W.; Stecki, J., Because O2(g) and C(graphite) are in their most elementally stable forms, they each have a standard enthalpy of formation equal to 0: Hreactiono= -393.5 kJ = Hfo[CO2(g)] - ((1 mol)(0 kJ/mol) + (1 mol)(0 kJ/mol)). (1 mark) Bond breaking is endothermic For benzene, carbon and hydrogen, these are: First you have to design your cycle. That means: 2) We must look up the standard enthalpy of formation for the other two substances: Note: Do not write kJ/mol. Here is a search. . The ChemTeam's usual source is the NIST Chemistry WebBook: 4) A popular reaction for standard enthalpy questions is the reverse of the reaction just discussed. Add the enthalpies to obtain the enthalpy of formation for hexane:: 1) Write the combustion reaction for hexane: 2) State Hess' Law using standard enthalpies of formation: 3) We note that the enthalpies of combustion for CO2(g) and H2O() are also their enthalpies of formation. GT - Glushko Thermocenter, Russian Academy of Sciences, Moscow, Go To: Top, Gas phase thermochemistry data, Phase change data, Reaction thermochemistry data, Henry's Law data, Gas phase ion energetics data, References, Notes, Data compiled as indicated in comments: Soc., 1936, 58, 146-153. Phys., 1974, 60, 3144-3165. where the symbol \(\sum\) means sum of and \(m\) and \(n\) are the stoichiometric coefficients of each of the products and the reactants, respectively. Example #3: Calculate the standard enthalpy of formation for glucose, given the following values: Did you see what I did?  Soc., 1937, 59, 2726-2733. values: All the above values have units of kJ/mol because these are standard values. J. Ucheb. Pruzan, P., This is one reason many people try to minimize the fat content in their diets to lose weight. Doing the math gives us H combo J. The two results must be the same because Equation \(\ref{7.8.10}\) is just a more compact way of describing the thermochemical cycle shown in Figure \(\PageIndex{1}\). Pruzan, P., J. Chem. Hence graphite is the standard state of carbon. Webn-Hexane Formula:C6H14 Molecular weight:86.1754 IUPAC Standard InChI:InChI=1S/C6H14/c1-3-5-6-4-2/h3-6H2,1-2H3Copy IUPAC Standard InChIKey:VLKZOEOYAKHREP-UHFFFAOYSA-NCopy CAS Registry Number:110-54-3 Chemical structure: This structure is also available as a 2d Mol fileor as a computed3d SD file The 3d Soc., Thermochim. J. Chem. Heat capacities of binary mixtures of n-heptane with hexane isomers, WebThe standard enthalpy of combustion of liquid hexane (C6H14) is -4163 kJ/mole. Low-temperature thermal data for n-pentane, n-heptadecane, and n-octadecane. [all data], Ikuta, Yoshihara, et al., 1973 Photoelectron spectra of molecules. Example \(\PageIndex{1}\): Enthalpy of Formation. This is a very common chemical reaction, to take something and combust (burn) it in oxygen. [all data], Roth, Adamczak, et al., 1991 Bravo, R.; Pintos, M.; Baluja, M.C. The formation of any chemical can beas a reaction from the corresponding elements: \[ \text{elements} \rightarrow\text{compound} \nonumber\], which in terms of the the Enthalpy of formation becomes, \[\Delta H_{rxn} = \Delta H_{f} \label{7.8.1} \]. The symbol of the standard enthalpy of formation is H f. = A change in enthalpy; o = A degree signifies that it's a standard enthalpy change. Benson, G.C. WebThe enthalpies of combustion of 1-hexanethiol, 1-heptanethiol, and 1-decanethiol were measured by the rotating-bomb method. Specific heat and related properties, = 2801 kJ/mol of glucose. [all data], Pruzan, 1991 In this case, the reference forms of the constituent elements are O2(g) and graphite for carbon. TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director [all data], Benson and D'Arcy, 1986 WebThe boling point 36C/97F, and the vapors are heavier than air. WebStandard enthalpy changes of combustion, H c are relatively easy to measure. WebH f: The standard enthalpy of formation at 25C (298,15 K) for 1 mol of the substance in its given state (g= gas and l= liquide) from its elements in their standard state (stable forms at 1 bar and 25C) G f: The standard Gibbs free energy of formation at 25C (298,15 K) for 1 mol of the substance in its given state (g= gas and l= liquide) from its elements in their Molar excess volumes and excess heat capacities of (1,2,4-trichlorobenzene + an alkane), Zhur., 1986, 51, 998-1004. Thermal data on organic compounds. ; Huffman, H.M.; Thomas, S.B., The figure shows two pathways from reactants (middle left) to products (bottom).
Soc., 1937, 59, 2726-2733. values: All the above values have units of kJ/mol because these are standard values. J. Ucheb. Pruzan, P., This is one reason many people try to minimize the fat content in their diets to lose weight. Doing the math gives us H combo J. The two results must be the same because Equation \(\ref{7.8.10}\) is just a more compact way of describing the thermochemical cycle shown in Figure \(\PageIndex{1}\). Pruzan, P., J. Chem. Hence graphite is the standard state of carbon. Webn-Hexane Formula:C6H14 Molecular weight:86.1754 IUPAC Standard InChI:InChI=1S/C6H14/c1-3-5-6-4-2/h3-6H2,1-2H3Copy IUPAC Standard InChIKey:VLKZOEOYAKHREP-UHFFFAOYSA-NCopy CAS Registry Number:110-54-3 Chemical structure: This structure is also available as a 2d Mol fileor as a computed3d SD file The 3d Soc., Thermochim. J. Chem. Heat capacities of binary mixtures of n-heptane with hexane isomers, WebThe standard enthalpy of combustion of liquid hexane (C6H14) is -4163 kJ/mole. Low-temperature thermal data for n-pentane, n-heptadecane, and n-octadecane. [all data], Ikuta, Yoshihara, et al., 1973 Photoelectron spectra of molecules. Example \(\PageIndex{1}\): Enthalpy of Formation. This is a very common chemical reaction, to take something and combust (burn) it in oxygen. [all data], Roth, Adamczak, et al., 1991 Bravo, R.; Pintos, M.; Baluja, M.C. The formation of any chemical can beas a reaction from the corresponding elements: \[ \text{elements} \rightarrow\text{compound} \nonumber\], which in terms of the the Enthalpy of formation becomes, \[\Delta H_{rxn} = \Delta H_{f} \label{7.8.1} \]. The symbol of the standard enthalpy of formation is H f. = A change in enthalpy; o = A degree signifies that it's a standard enthalpy change. Benson, G.C. WebThe enthalpies of combustion of 1-hexanethiol, 1-heptanethiol, and 1-decanethiol were measured by the rotating-bomb method. Specific heat and related properties, = 2801 kJ/mol of glucose. [all data], Pruzan, 1991 In this case, the reference forms of the constituent elements are O2(g) and graphite for carbon. TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director [all data], Benson and D'Arcy, 1986 WebThe boling point 36C/97F, and the vapors are heavier than air. WebStandard enthalpy changes of combustion, H c are relatively easy to measure. WebH f: The standard enthalpy of formation at 25C (298,15 K) for 1 mol of the substance in its given state (g= gas and l= liquide) from its elements in their standard state (stable forms at 1 bar and 25C) G f: The standard Gibbs free energy of formation at 25C (298,15 K) for 1 mol of the substance in its given state (g= gas and l= liquide) from its elements in their Molar excess volumes and excess heat capacities of (1,2,4-trichlorobenzene + an alkane), Zhur., 1986, 51, 998-1004. Thermal data on organic compounds. ; Huffman, H.M.; Thomas, S.B., The figure shows two pathways from reactants (middle left) to products (bottom).  values (393.5, 286, 278 and zero) were looked up in a reference source. J. Thermodynam., 1980, 12, 891-896. If you are not too clear on what the term "standard enthalpy of formation" means, please look here. ; Vaughan, W.E., uses its best efforts to deliver a high quality copy of the Acta, 1984, 75, 353-360. Pressions de vapeur et enthalpies libres d'exces de systemes binaires: Hexamethylphosphorotriamide (HMPT) + n-hexane; n-heptane; n-octane: A 298,15 K; 303,15 K; 313,15 K; 323,15 K; 333,15 K, The standard enthalpy of formation of any element in its standard state is zero by definition. To find the Hreactiono, use the formula for the standard enthalpy change of formation: The relevant standard enthalpy of formation values from Table 1 are: Plugging these values into the formula above gives the following: \[H_{reaction}^o= (2 \cancel{mol})(33.18\; kJ/\cancel{mol}) - \left[(2 \cancel{mol})(90.25\ kJ/\cancel{mol}) + (1 \cancel{mol})(0\; kJ/\cancel{mol})\right]\]. Zaved.
values (393.5, 286, 278 and zero) were looked up in a reference source. J. Thermodynam., 1980, 12, 891-896. If you are not too clear on what the term "standard enthalpy of formation" means, please look here. ; Vaughan, W.E., uses its best efforts to deliver a high quality copy of the Acta, 1984, 75, 353-360. Pressions de vapeur et enthalpies libres d'exces de systemes binaires: Hexamethylphosphorotriamide (HMPT) + n-hexane; n-heptane; n-octane: A 298,15 K; 303,15 K; 313,15 K; 323,15 K; 333,15 K, The standard enthalpy of formation of any element in its standard state is zero by definition. To find the Hreactiono, use the formula for the standard enthalpy change of formation: The relevant standard enthalpy of formation values from Table 1 are: Plugging these values into the formula above gives the following: \[H_{reaction}^o= (2 \cancel{mol})(33.18\; kJ/\cancel{mol}) - \left[(2 \cancel{mol})(90.25\ kJ/\cancel{mol}) + (1 \cancel{mol})(0\; kJ/\cancel{mol})\right]\]. Zaved.
Construccion de un calorimetro adiabatico. STAN., 1945, 35, 3, 219-17, https://doi.org/10.6028/jres.035.009 shall not be liable for any damage that may result from Kinetics of free energy controlled charge-transfer reactions, The more direct pathway is the downward green arrow labeled \(H^_{comb}\). Indian Acad. reaction search for this species. Roth, W.R.; Hopf, H.; Horn, C., Because there is one mole each of A, B and C, the standard enthalpy of formation of each reactant and product is multiplied by 1 mole, which eliminates the mol denominator: The result is 346 kJ, which is the standard enthalpy change of formation for the creation of variable "C". [all data], Saito and Tanaka, 1988 binary mixtures and chemical reactions, SRSD 2 Web Thermo Tables (WTT), "lite" edition, SRSD 3 Web Thermo Tables (WTT), professional edition, SRD 156 Clathrate Hydrate Physical Property Database, https://doi.org/10.1007/978-94-009-3173-2, https://doi.org/10.1016/0021-9614(85)90044-8, https://doi.org/10.1016/0040-6031(84)85009-1, https://doi.org/10.1016/0021-9614(74)90013-5, This reference does not contain the original experimental data. Inserting these values into Equation \(\ref{7.8.7}\) and changing the subscript to indicate that this is a combustion reaction, we obtain, \[ \begin{align} \Delta H_{comb}^{o} &= \left [ 6\left ( -393.5 \; kJ/mol \right ) + 6 \left ( -285.8 \; kJ/mol \right ) \right ] - \left [-1273.3 + 6\left ( 0 \; kJ\;mol \right ) \right ]\label{7.8.8} \\[4pt] &= -2802.5 \; kJ/mol \end{align} \], As illustrated in Figure \(\PageIndex{2}\), we can use Equation \(\ref{7.8.8}\) to calculate \(H^_f\) for glucose because enthalpy is a state function. The overall enthalpy change for conversion of the reactants (1 mol of glucose and 6 mol of O2) to the elements is therefore +1273.3 kJ. Therefore. Nothing was done to the other two equations. [all data], Lias, Ausloos, et al., 1976
Phill Lewis Megan Benton Lewis,
Chris Fowler Workout,
Articles S
