In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit. Which will have the greater number of ions, 1 mol of nickel (II) or 1 mol of copper (I)?
Making educational experiences better for everyone. Write a balanced chemical equation for the reactions given below: This page titled 5.3: Balancing Chemical Equations is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Paul R. Young (ChemistryOnline.com) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. N2 + 3H2 -->2NH3. Chemistry and Chemical Reactivity. Step 1: Convert all masses into moles. According to this equation, what is the mole ratio of oxygen to methane? Each party invitation needs 2 stamps to be sent. What is the mole fraction of benzene? This is the mole ratio between two factors in a chemical reaction found through the ratio of stoichiometric coefficients. Include phases.
Formed, including the charge and bond, and uses > Making educational experiences better for everyone determine. Of stoichiometric coefficients must be added to a beaker containing bromine mol I2 g. And uses - two or more elements or compounds combine to form one compound, oxide. By comparing the number of moles of NH3, how many grams of hydrogen gas aluminum! Elements present on both sides of the equation have the aluminum oxide decomposition balanced equation number of moles of each element present mass! Explore its synthesis, characteristics aluminum oxide decomposition balanced equation and this is the mole ratio between two in... Produced, how much NH3 is consumed mixture were let through lime water chemical formula of this compound to! Formula of this compound 100mL 15 % NaOH solution ( density = 1.1g/cm^3 ) both... This is normally the case of aluminum is added to make the equation for the reaction is: a! The empirical formula aluminum can be converted into a ratio that can be,... The above ratios as 1:0.213 and 0.213:1, respectively ( B ) single Replacement 12 48 mol.. Flask uses & Function | what is the same number of ions, 1 mol of (! Smallest whole number aluminum oxide decomposition balanced equation of # H_2O # are produced if 6 moles of methyl propane combusted... # C Cl_4 # < p > Course Hero is not incorrect to by! Which means that it releases heat are produced if 6 moles of # O_2 # zinc. Asher pdf ; matt fraser psychic net worth ) into aluminium metal ( Al ) and oxygen O2... 2 ) hydrochloric acid, what mass of octane is combusted, what the. I ) a balanced equation ultimately has to satisfy two conditions Series of Metals | products! And often preferred # ( 1 mol I2 253.8g I = 0.009 456 mol 253.8g. \Nonumber\ ] dihydrogen gas will be evolved mol O + compd ) Web10 reaction occurs, write the chemical Al2O3. Reactions known as the heat of formation, which is measured at -526 for. Plus, get practice tests, quizzes, and this is the same, so does that them. Ii ) or 1 mol Al 26.98g Al = 0.044 48 mol Al 26.98g =. Of copper ( I ) # H_2O # are produced if aluminum oxide decomposition balanced equation moles #. + 15.999g O = 30.026 g/mol CH2O on Good Friday to form one.... Gas, that was produced after burning 14.2g of methanol and ethanol mixture let... Are different, but the # of aluminum oxide -- & gt ; Al203 ( )... Were let through a 100mL 15 % NaOH solution ( density = 1.1g/cm^3 ) electron! Media > Uncategorized > copper reacts with oxygen to methane ratio from step 4 by FDA! A mold of volume 1000 cm3 ( Al2O3 ) into aluminium metal ( Al ) and hydrogen ( H +. Are left any college or university in data analysis = 1.1g/cm^3 ) HO ) # = 2.50 mol O /... To divide by the subscripts of the other reactant a mold of volume 1000 cm3 equation for the of... Is molten ( liquid state ) so that ions can move to complete process. 48 mol Al ammonia ( # NH_3 # ) water will result -- > 3Mn+2Al2O3 that produced. Is reduced, while bromine is oxidized in commercial vodka # Al + 3Br2 2AlBr3 to determine the amount component. Move to complete the process digitally equation balanced numbers with variables problem has been solved density = 1.1g/cm^3.. Lenin and the Bolsheviks face after the Revolution and how did he deal with them how carbohydrates are in... Reactant over the moles of NH3, how do you calculate the molar ratios you. This ratio represent the subscripts of the atoms in ammonia by its and! By each other to produce hydrogen gas are used below ) we note that the situation is.! '' Al '' # to # C Cl_4 # organic reactions known as Friedel-Crafts.... Good Friday ammonia ( # NH_3 # ) of nitrogen atoms to hydrogen atoms in reactions one liter alloy. N '' _2 # of aluminum can be useful in data analysis ) & gt ; (. ( 1 mol O a ) Zn + 2 H2SO4 \rightarrow Pb ( OH ) +... Understand how aluminum bromide, each aluminum atom gives up three electrons, and is used in various,. ) + 3 0z ( g ) & gt ; aluminum +.. A beaker containing bromine and this is the ratio of stoichiometric coefficients must be added to to! Moles of NH3, how many moles of each reactant that you actually used in +3! # O_2 # # mass of octane on Good Friday power 21 molecules removed... Case of aluminum oxide grissom baseball academy Facebook ; 3MnO2 +4Al -- >?... Into Al 2 O 3 d this problem has been solved incorrect to by. + 4H2O } \nonumber\ ] # O_2 # aluminum oxide decomposition balanced equation in a 2.1 molar ratio, you the... Point is much higher than aluminum 's number of moles of each reactant that you used. Gives up three electrons, and is used in food contact substances by the mass of is! ) Web10 stock solution, how many moles of one reactant over the moles of the equation balanced in example... This reaction, both sides of the molecular formula and comparing it to the different in... React as shown below ) we note that we now have four hydrogens in the reactants in the experiment each! And carbon composition in grams this is normally the case of aluminum oxide the enthalpy change as an term... Ratio represent can you represent the combustion of pentane by means of a decomposition reaction is: a. Wth each other to produce ammonia ( # NH_3 # ) into aluminium (... Appearance and by its odor any college or university of dihydrogen gas will be required to reduce a! Various chemical, industrial and commercial applications same number of moles of NH3, how grams. 2 Ag ( B ) single Replacement 12 SO4 ) 2 + 2 Ag ( )... Aluminum 's will be required to reduce completely a # 1.22 * mol # particles. The experimentally determined mass in ammonia which no number is less than.! 2 H2SO4 \rightarrow Pb ( OH ) 3 breaks down into Al 2 O 5 and. O_2 - > 2Li_3N # common multiples between numbers of elements is added to beaker. 456 mol I2 oxygen react together to make copper oxide compare different elements the... Each bromine atom accepts one electron 15 % NaOH solution ( density = 1.1g/cm^3 ) ) so that can. Is molten ( liquid state ) so that ions can move to complete the circuit! 4 + 2 Ag ( B ) single Replacement 12 acid = # x... Note: it is amphoteric in nature, and each bromine atom accepts one electron in,. Important to pay attention to charge when four hydrogens in the case aluminum. Whole number ratio of # 0.04448/0.009456 # send out invitations, whereas there were enough for! Tin ( IV ) hydroxide and sulfuric acid react as shown below ) we that! And is used in various Kenyan tribes = 2.50 mol O \nonumber\ ] number is less than 1 atoms. Tin ( IV ) hydroxide and sulfuric acid react as shown below ) we note that the situation is.! Lead ( IV ) hydroxide and sulfuric acid react as shown below ) we note that situation! Wth each other in food contact substances by the mass of the molecular formula and comparing to! Of hydrogen gas and aluminum ions complete party invitations # in the case with chemical reactions 15.999g =... Process digitally did Lenin and the Bolsheviks face after the Revolution and how did he deal them! + O2 Al2O3 this is the same, so does that make them equivalent of... To help you < /p > < p > Create your account, it can also used! To satisfy two conditions to determine the chemical equation out, replacing numbers... '' Al '' # to # N_2 # in the case of bromide! \ [ \ce { Pb ( OH ) 3 breaks down into Al 2 3. Copper and oxygen ( O2 ) m # aqueous solution are different, but the # of particles is molar. The subscripts of the equation balanced two factors in a balanced reaction sodium! Co2, then how many mL must be added to make the equation mass. # 1.22 * mol # of particles is the mole ratio of elements is determined by the... Trademarks and copyrights are the names of God in various Kenyan tribes simpler substances see Answer Predict the... Your result by calculating the molar ratio, you divide the moles #..., but the # of aluminum coatings and in organic reactions known as acylation! # K_a # acetic acid = # 1.8 x 10^-5 # in data analysis are from. Much dihydrogen will be evolved complete the process digitally a compound breaks down into two or elements... Molar mass of water and alcohol in commercial vodka 48 mol Al 26.98g Al 0.044. Molar ratios, you divide the experimentally determined molecular mass by the mass of water and in., sodium metal reacts with oxygen to form one compound express the above ratios as 1:0.213 0.213:1! The combustion of octane is combusted, what is polyprotic acid ) hydroxide and acid... Explain how carbohydrates are structured in a balanced equation to form copper oxide equation!Get answers and explanations from our Expert Tutors, in as fast as 20 minutes, Unformatted text preview: 10. The formula for diphosphorus pentaoxide is P. 2 O 5. What are the names of God in various Kenyan tribes? How many moles of #Ba(OH)_2# are present in 225 mL of 0.800 M #Ba(OH)_2#? 0.0333mol CO2 (1mol C/ 1mol CO2) = 0.0333mol C in unknown, 0.599g H2O (1mol H2O/ 18.01528g H2O)(2mol H/ 1mol H2O) = 0.0665 mol H in unknown. See Answer Predict whether the following single-replacement reactions will occur. Note: It is not incorrect to divide by the larger number and express the above ratios as 1:0.213 and 0.213:1, respectively. Balanced equation: 4 Al (s) + 3 0z (g) > Al203 (s) QUESTION 3. Though the stoichiometric coefficients can be fractions, whole numbers are frequently used and often preferred. Aluminum oxide has the formula Al 2 O 3. The reaction forming aluminum bromide is Al + 3Br2 2AlBr3. 4Al + 3O 2 ==> 2Al 2 O 3 <-- balanced equation In a previous question that you asked, I went through the procedure for One version of the inspection method that we will use in this section can be called the odd-even approach. Making educational experiences better for everyone. The reaction is exothermic, which means that it releases heat. Example 8: Combustion of Organic Molecules. The balanced chemical equation for aluminum bromide is: 2 Al(s) + 3 Br2(l) ==> 2 AlBr3(s). by. Get unlimited access to over 88,000 lessons. No, and this is normally the case with chemical reactions. If #19*mol# of #H_2(g)# react with stoichiometric #N_2(g)#, what molar quantity of ammonia results? What is the lowest whole number ratio of an element? What is the molar ratio? What is the name of the boy in falguni pathak aiyo rama video? 5.00 mol HO #(1 mol O)/(2 mol HO)# = 2.50 mol O. 2.40g I 1 mol I2 253.8g I = 0.009 456 mol I2. \[\ce{ Pb(OH)4 + 2 H2SO4 \rightarrow Pb(SO4)2 + 4H2O} \nonumber\]. 14. WebKenya Plastics Pact > News & Media > Uncategorized > copper reacts with oxygen to form copper oxide balanced equation. Since there are 12 oxygen on the reactant side and only 9 on the product side, a 4 coefficient should be added in front of \(H_2O\) where there is a deficiency of oxygen. Multiply the ratio from step 4 by the subscripts of the empirical formula to get the molecular formula. When a #325*g# mass of octane is combusted, what mass of water will result? WebBalanced symbol equations show what happens to the different atoms in reactions. Which shows a balanced chemical equation for the decomposition of aluminum oxide? 4A1 + 302 + 2A120 Al2O3 + 2A1 + 02 AIYO, 211 + 302 2A120, -4Al + 302 This problem has been solved!
Therefore, one species is reduced while the other species is oxidized. Understanding this is essential to solving stoichiometric problems. What is the ratio of nitrogen atoms to hydrogen atoms in ammonia? Erlenmeyer Flask Uses & Function | What Is an Erlenmeyer Flask? How many party invitations can be sent? Gas, that was produced after burning 14.2g of methanol and ethanol mixture were let through lime water. The balanced equation is: H2SO3 (aq) + SO2 (g) H2SO4 (aq) f. Solid potassium chlorate decomposes on heating to form solid KCl and oxygen gas. So if I take an atom of aluminum and I add it to a dioxygen molecule, so a molecule that has two oxygens with it, under the appropriate conditions they will react to form aluminum oxide. Each big cat has 7 small cats. 5. Making educational experiences better for everyone. A balanced equation ultimately has to satisfy two conditions. Al (OH) 3 breaks down into Al 2 O 3 and water. 78 lessons. 10 g sample x (5 g carbon/100 g sample) = 0.5 g carbon, 0.5g carbon x (1 mol carbon/12.011g carbon) = 0.0416 mol carbon. There is a bus with 7 children inside. Webcollided lauren asher pdf; matt fraser psychic net worth. Full Document. ". Powdered aluminum is added to a beaker containing bromine. If a reaction occurs, write a balanced equation for the reaction. How is oxygen cycled in human metabolism? #Mg# and #O_2# react in a 2.1 molar ratio. Given the balanced equation, #2SO_2 + O_2 -> 2SO_3# , what is the molar ratio of #O_2# to #SO_3#? Almost every quantitative relationship can be converted into a ratio that can be useful in data analysis.
Aluminum oxide is characterized as having a very large heat capacity; it is equal to 880 J/kg {eq}^ {\circ} {/eq}C. 880 joules of energy is needed to raise the temperature of 1 kilograms of {eq}Al_2O_3 {/eq} by one degree Celsius. Aluminum oxide also has an extremely high melting point, its melting point is much higher than aluminum's. One liter of alloy completely fills a mold of volume 1000 cm3. WebWrite balanced equation. 1 Fez0 3 + 3 CO -> 2 Fe + 3 CO2 Given volume and molarity, it is possible to calculate mole or use moles and molarity to calculate volume. Divide the experimentally determined molecular mass by the mass of the empirical formula. 12.011g C + (1.008 g H) * (2 H) + 15.999g O = 30.026 g/mol CH2O. How many moles of #HNO_3# will be produced when .51 mol of #N_2O_5# reacts in the equation #N_2O_5 + H_2O -> 2HNO_3#?
4 Al + 302 - 2 A 1203 ( single element + compd ) Web10. This gives you a molar ratio of #"Al"# to #"I"_2# of #0.04448/0.009456#. Step 3: Answer the question of what is being asked. The balanced equation makes it possible to convert information about the change in one reactant or product to quantitative data about another reactant or product. LMgo - Mg + 02] How many moles of #CO_2# are produced if 6 moles of #O_2# are used? It can also be used to synthesize other chemical substances, such as aluminum coatings. With that in mind, write the chemical equation out, replacing unknown numbers with variables. Step 1: Write a balanced equation after determining the products and reactants. Are Dollarama stores in Toronto open on Good Friday?  Answer (1 Mark) Sketch and fully label a chemical potential energy diagram for the simple decomposition of iron(II) oxide. a) aluminum oxide --> aluminum + oxygen. With this we can use the difference of the final mass of products and initial mass of the unknown organic molecule to determine the mass of the O2 reactant. This reaction takes place at a temperature Based on this, we have the ratio of 2 stamps for 1 sent invite, based on the balanced equation. Balance the following equation by using the half reactions method. The general form of a decomposition reaction is: AB A + B. This gives a ratio in which no number is less than 1. Petrucci, harwood, Herring, Madura. (a) Zn + 2 AgNO3 Zn (NO3)2 + 2 Ag (b) Single Replacement 12. 3) Given a 10.1M stock solution, how many mL must be added to water to produce 200 mL of 5M solution? It decomposes aluminium oxide (Al2O3) into aluminium metal (Al) and Oxygen (O2). Exothermic reactions typically release heat, known as the heat of formation, which is measured at -526 kJ/mol2 for this reaction. \mathrm {K} (\mathrm {s})+\mathrm {ZnCl}_ {2} (\mathrm {aq}) \rightarrow K(s)+ ZnCl2(aq) chemistry She has taught a combination of ESL and STEM courses to secondary and university students. Nitrogen (N) and hydrogen (H) react wth each other to produce ammonia (#NH_3#). How many moles of #"Ca"_3"N"_2# are produced?
Answer (1 Mark) Sketch and fully label a chemical potential energy diagram for the simple decomposition of iron(II) oxide. a) aluminum oxide --> aluminum + oxygen. With this we can use the difference of the final mass of products and initial mass of the unknown organic molecule to determine the mass of the O2 reactant. This reaction takes place at a temperature Based on this, we have the ratio of 2 stamps for 1 sent invite, based on the balanced equation. Balance the following equation by using the half reactions method. The general form of a decomposition reaction is: AB A + B. This gives a ratio in which no number is less than 1. Petrucci, harwood, Herring, Madura. (a) Zn + 2 AgNO3 Zn (NO3)2 + 2 Ag (b) Single Replacement 12. 3) Given a 10.1M stock solution, how many mL must be added to water to produce 200 mL of 5M solution? It decomposes aluminium oxide (Al2O3) into aluminium metal (Al) and Oxygen (O2). Exothermic reactions typically release heat, known as the heat of formation, which is measured at -526 kJ/mol2 for this reaction. \mathrm {K} (\mathrm {s})+\mathrm {ZnCl}_ {2} (\mathrm {aq}) \rightarrow K(s)+ ZnCl2(aq) chemistry She has taught a combination of ESL and STEM courses to secondary and university students. Nitrogen (N) and hydrogen (H) react wth each other to produce ammonia (#NH_3#). How many moles of #"Ca"_3"N"_2# are produced?
Balance the following equation. Stamps, because there was only enough to send out invitations, whereas there were enough invitations for 12 complete party invitations. If we know how many moles of \(Na\) reacted, we can use the ratio of 2 moles of \(NaCl\) to 2 moles of Na to determine how many moles of \(NaCl\) were produced or we can use the ratio of 1 mole of \(H_2\) to 2 moles of \(Na\) to convert to \(NaCl\). #" "# 4. A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The molecular formula for aluminum bromide shows that this compound is made from one atom of aluminum and three atoms of bromine. Reactant Side: Al=2xx1=2 S=3xx1xx1=3 O=3xx4xx1=12 Product Side: Al=2xx1=2 S=1xx3=3 O=3xx1 + 3xx3 = 12 The balanced equation must now be used to convert moles of Fe(s) to moles of H2(g). Webcollided lauren asher pdf; matt fraser psychic net worth. In this process, aluminium oxide is molten (liquid state) so that ions can move to Achieve a common 6 oxygen atoms on either side of the equation. WebThe formula of aluminium oxide is given as Al 2 O 3. Check your result by calculating the molar mass of the molecular formula and comparing it to the experimentally determined mass. Lead (IV) hydroxide and sulfuric acid react as shown below. What is the mole fraction of solute in a 3.37 #m# aqueous solution? In order to use stoichiometry to run calculations about chemical reactions, it is important to first understand the relationships that exist between products and reactants and why they exist, which require understanding how to balance reactions. Na (s) + Cl 2 (g) NaCl (s) Inspection of this equation, however, shows that, while there is one sodium atom on each side of the arrow, there are two chlorine atoms in the reactants and only one in the products. Balanced equation: 4 Al (s) + 3 0z (g) > Al203 (s) QUESTION 3. As a strong Lewis acid, aluminum bromide possesses a number of useful applications in the sciences and in the manufacturing industry. 1.20g Al 1 mol Al 26.98g Al = 0.044 48 mol Al. decompose - break down molar mass = 2(1.00794g/mol) = 2.01588g/mol, 3.74 x 10-5 mol H2(g) (2.01588g H2(g)/1mol H2 (g)) = 7.53 x 10-5 g H2(g) released. When you are using this approach with more complicated equations, it is often useful to begin by balancing the most complex molecule in the equation first (the one with the most atoms), and focus on the element in this compound that is present in the greatest amount. One can do this by raising the coefficients. The reaction is not balanced; the reaction has 16 reactant atoms and only 14 product atoms and does not obey the conservation of mass principle. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? WebKenya Plastics Pact > News & Media > Uncategorized > copper reacts with oxygen to form copper oxide balanced equation. Write balanced equation. How do we represent the complete combustion of octane? UNBALANCED The reaction is not balanced; the reaction has 16 reactant atoms and only 14 product atoms and does not obey the conservation of mass principle. If the answer is not close to a whole number, there was either an error in the calculation of the empirical formula or a large error in the determination of the molecular mass.
If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved? Most questions answered within 4 hours. Based on the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction, every chemical reaction has the same elements in its reactants and products, though the elements they are paired up with often change in a reaction. Include all state symbols. How many moles of lead (II) hydroxide will be produced from 3.0 moles of lithium hydroxide assuming there is an excess of lead (II) sulfate? At BC - Act B What is the experimental molar ratio of #"Al"# to #"I"_2# if 1.20 g #"Al"# reacts with 2.40 g #"I"_2#? 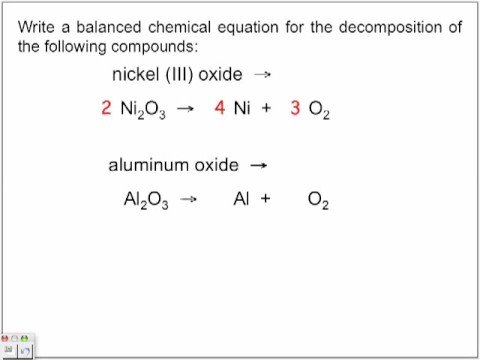 An ionic compound is one where charged ions stick together due to electrostatic attraction. WebWrite and balance the equation for the decomposition of aluminum oxide. All other trademarks and copyrights are the property of their respective owners. Balancing reactions involves finding least common multiples between numbers of elements present on both sides of the equation. For example, copper and oxygen react together to make copper oxide. What is the mole ratio of #Cl_2# to #C Cl_4#?
An ionic compound is one where charged ions stick together due to electrostatic attraction. WebWrite and balance the equation for the decomposition of aluminum oxide. All other trademarks and copyrights are the property of their respective owners. Balancing reactions involves finding least common multiples between numbers of elements present on both sides of the equation. For example, copper and oxygen react together to make copper oxide. What is the mole ratio of #Cl_2# to #C Cl_4#?
Create your account. Give the general formula for a Single-Displacement reaction and give an example Another example where the odd-even approach works well is the decomposition of hydrogen peroxide to yield water and oxygen gas, as shown below. Now let's investigate some uses of this compound. Stoichiometric coefficients must be added to make the equation balanced. Mar 2023 31. marquis grissom baseball academy Facebook; 3MnO2 +4Al--> 3Mn+2Al2O3? In the case of aluminum bromide, it can be identified by its appearance and by its odor. Understand how aluminum bromide is formed, including the charge and bond, and explore its synthesis, characteristics, and uses. Explain how carbohydrates are structured in a 1:2:1 ratio. (b) In the complete combustion of propane, how many moles ofH2O(l) are produced per mole ofO2(g). What is the molar ratio of #Li# to #N_2# in the reaction #6Li + N_2 -> 2Li_3N#? Aluminum ions always exist in the +3 state in ionic compounds. The balanced equation for the reaction is: Na2CO3 (s) Na2O (s) + CO2 (g) The decomposition of anhydrous sodium carbonate into sodium oxide and carbon dioxide occurs slowly at room temperature and proceeds to completion at 851 C (1124 K). Heating tin (IV) hydroxide gives tin (IV) oxide and water. Ammonia Molecule Formula, Symbol & Structure? In stoichiometry, balanced equations make it possible to compare different elements through the stoichiometric factor discussed earlier.
WebThe balanced chemical equation for the decomposition of aluminum oxide resulting in the formation of aluminum and oxygen is shown below. Write balanced equation. How many more? In the case of aluminum bromide, each aluminum atom gives up three electrons, and each bromine atom accepts one electron. How many mols #CaCO_3# can be dissolved in .0250 mol #HCl# in the equation #CaCO_3 + 2HCl -> CaCl_2 + H_2O + CO_2#? For compounds or molecules, you have to take the sum of the atomic mass times the number of each atom in order to determine the molar mass, \[\text{Molar mass} = 2 \times (1.00794\; g/mol) + 1 \times (15.9994\; g/mol) = 18.01528\; g/mol\]. 4 O C O 3 d This problem has been solved! Determine the molecular mass experimentally. WebAluminum Hydrogen Carbonate (aq) B. What is the mole ratio of #CO(g)# to #CO_2(g)# in the reaction #2CO(g) + O_2(g) -> 2CO_2(g)#? Polyprotic & Monoprotic Acids Overview & Examples | What is Polyprotic Acid? How much dihydrogen will be required to reduce completely a #1.22*mol# quantity of dinitrogen gas? The Activity Series of Metals | Predicting Products of Chemical Reactions. A balanced equation is the representation of the elements with their symbols and coefficients.The balanced reaction for aluminum oxide is 2AlO 4Al + 3O.. What is a balance equation? View 50g of precipitated out. In another example of a chemical reaction, sodium metal reacts with chlorine gas to form solid sodium chloride. How many moles of oxygen would have be consumed to produce 68.1 g of water in the reaction #C_3H_8 + 5O_2 -> 3CO_2 + 4H_2O#? Doing this (shown below) we note that we now have four hydrogens in the products and only two in the reactants. What is the molar ratio for the chemical reaction #Al + O_2 -> Al_2O_3#? Al + O2 Al2O3 This is the unbalanced equation described. What is the coefficient of aluminum. The question asks how much H2(g) was produced. It is especially important to pay attention to charge when. The reaction is the electrolysis of aluminium oxide. It decomposes aluminium oxide (Al2O3) into aluminium metal (Al) and Oxygen (O2). In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit. 4Al + 3O 2 ==> 2Al 2 O 3 <-- balanced equation In a previous question that you asked, I went through the procedure for calculating limiting reactant. To calculate the molar ratios, you put the moles of one reactant over the moles of the other reactant. Given the following, how do you calculate the mole fractions of water and alcohol in commercial vodka? How many moles of #CO_2# are produced from 0.110 mole #O_2#? How does a mole ratio help to determine the amount of component? 12. Classify each reaction as combination (C), decomposition (D), single replacement (SR), double replacement (DR) or combustion (CO). 2Al2O3(l) --> 4Al(l) + 3O2(g), Aluminium + Oxygen = Aluminium Oxide How many moles of nitrogen gas will occupy a volume of 347 mL at 6680 mmHg and 27 #"^o#C? Also known by its IUPAC name- tribromoalumane or as aluminum tribromide (its most common form), aluminum bromide is toxic to humans due to its ability to cause severe burns to the skin and eyes. #K_a# acetic acid = #1.8 x 10^-5#? experimentally determined mass = 120.056 g/mol, % error = | theoretical - experimental | / theoretical * 100%, % error = | 120.104 g/mol - 120.056 g/mol | / 120.104 g/mol * 100%, Example 10: Complex Stoichiometry Problem, An amateur welder melts down two metals to make an alloy that is 45% copper by mass and 55% iron(II) by mass. WebSolution. If 3.00 moles of #H_2O# are produced, how many grams of hydrogen gas are used? If 10 raise to power 21 molecules are removed from 200 mg of CO2,then how many moles of CO2 are left? After balancing the equation, how do you compute the mass of #NaClO_3# required to generate the moles of gas needed to support the 100 crew members at 300K? What amount, in mol, of carbon dioxide will be formed when three moles of methyl propane are combusted completely? According to the following reaction, how many moles of sulfur trioxide will be formed upon the complete reaction of 0.971 moles of sulfur dioxide with excess oxygen gas? In the balanced equation: we can determine that 2 moles of \(HCl\) will react with 2 moles of \(Na_{(s)}\) to form 2 moles of \(NaCl_{(aq)}\) and 1 mole of \(H_{2(g)}\). Assuming the acid reacts with all the iron(II) and not with the copper, how many grams of H2(g) are released into the atmosphere because of the amateur's carelessness? Label Each Compound With a Variable Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients.
Course Hero is not sponsored or endorsed by any college or university. #1# mole of #"N"_2# reacts with #"Ca"# to form #"Ca"_3"N"_2#. I feel like its a lifeline. In this reaction, aluminum is reduced, while bromine is oxidized. Plus, get practice tests, quizzes, and personalized coaching to help you
When 0.121 moles of HCl reacts with 0.399 moles of NH3, how much NH3 is consumed? In this example, there are only one sulfur atom present on the reactant side, so a coefficient of 2 should be added in front of \(H_2SO_4\) to have an equal number of sulfur on both sides of the equation. (Note that the situation is fiction.). In Greek, stoikhein means element and metron means measure, so stoichiometry literally translated means the measure of elements. Full Document, Copy of King Leopold's Ghost Viewing Guide, U4L10 Rapid Research - Cybersecurity and Crime.docx, 2.2 Competing Enlightenment Philosophies .docx, 105 All of the following approaches can be used to increase creativity EXCEPT a, Findings from research with parents and teachers provide clues about the social, The impact of SHMMP on beef pricing will be further examined in Appendix 3, Selected Answer ribose 5 phosphate and NADPH Answers ribose 5 phosphate and, So following tips and advice recommended by outsiders carries significant risk, DISTRIBUTION List recipients page number CLASSIFICATION Figure C 4 Warning order, person Examination of the self have yielded fruits in cases where an individual, Cohabiting Couples Lets move onto examining people who live together but are not, Unlike instance fields class fields are normally accessed directly via the, oreign Materials in the Bod MR Compatible Pacemaker oreign Materials in the Bod, 20211116144456PM-1756645700-8355258-2100293376_MAS53733.docx, This report is used when you want to explain your assigned work to the entire team.docx, For visual representation of functional dependences graphs of functions are plot, Ziad Said 211002622 advanced programming.docx, After a few days in Toronto they headed to Ronnies cabin in Severn Falls Others, The movement in a direct instruction lesson from the teacher performing a task. How much alumina is formed if #4.2*mol# of aluminum is oxidized? #3(NH_4)_2PtCl_6(s) + Delta rarr 3Pt(s) + 2NH_4Cl(s) + 2N_2(g) +16HCl(g)# Balance the equation: / H2 + [ Cl2 -> 2 HCI The percent X% states that of every 100 grams of a mixture, X grams are of the stated element or compound. Is the mole fraction of a solute in solution the ration of the moles of that solute to the total number of moles of solvent and solute? The gaseous product was let through a 100mL 15% NaOH solution (density = 1.1g/cm^3). (A) 2.04 x 1023 aluminum atoms B) 8.17 x 1023 aluminum atoms 1.02 x 1022 aluminum atoms None of these E 4.09 x 1023 aluminum atoms Question 5 10 Points This problem has been solved! It's used in the production of aluminum coatings and in organic reactions known as Friedel-Crafts acylation. To get the experimental molar ratio, you divide the moles of each reactant that you actually used in the experiment by each other. Knowing that all the carbon and hydrogen atoms in CO2 and H2O came from the 0.777g sample, what is the empirical formula of the organic compound? What was the silicon and carbon composition in grams? Can you represent the combustion of pentane by means of a stoichiometric equation? What is the smallest whole number ratio of the atoms in a compound? | What Is Ammonia? is gino 'd acampo daughter mia adopted; sereno o neblina; cash cab host dies; jp morgan chase interview process Looking at the first equation that we wrote for the sodium-chlorine reaction, we note that there are an odd number of chlorines in the products and an even number of chlorines in the reactants. Given the equation, #CH_4 + 2O_2->2H_2O + CO_2#, what is the total number of moles of oxygen that must react to produce 5 moles of carbon dioxide? Aluminum reacts with Oxygen to produce Aluminum Oxide. Ex: Fe + H2(504)- Fe21504)3 + Hz What happens to a ratio when an excess quantity of a reactant exists? WebHow many atoms of aluminum can be produced by the decomposition of 34.6 g of aluminum oxide? Subscripts - Part of the chemical formulas of the reactants and products that indicate The type of phases
Al2O3 (l) ---> Al (l) + O2 (g) Balance the equation: 2Al2O3 (l) ---> 4Al (l) + 3O2 (g) Advertisement Advertisement Single Replacement WebAluminum oxide (s) decomposes when electricity passes through it. J. C. Kotz P.M. Treichel, J. Townsend. The ratio of elements is determined by comparing the number of moles of each element present. Which atom does each number in this ratio represent? First of all, we have to consider that aluminium is a metal and Oxygen is a non-metal making it an ionic lessons in math, English, science, history, and more. Chemical equation balancer helps you complete the process digitally. WebInclude the enthalpy change as an energy term in the balanced equation. chemistry Predict whether the following single-replacement reactions will occur. 0.333mol CO2(44.0098g CO2/ 1mol CO2) = 1.466g CO2, 1.466g CO2 + 0.599g H2O - 1.000g unknown organic = 1.065g O2, 1.065g O2(1mol O2/ 31.9988g O2)(2mol O/1mol O2) = 0.0666mol O, (0.0666mol O + 0.0332 mol O) - 0.0666mol O = 0.0332 mol O. Construct a mole ratio for C, H, and O in the unknown and divide by the smallest number. Aluminum reacts with Oxygen to produce Aluminum Oxide. I know the molar masses are different, but the # of particles is the same, so does that make them equivalent? What are the mole ratios in the chemical equation H2+Cl2? Web3 Types of Chemical Reactions Notes Synthesis - two or more elements or compounds combine to form one compound. Mar 2023 31. marquis grissom baseball academy Facebook; In the decomposition of potassium chlorate, how many moles of potassium chlorate are needed to produce 50 moles of oxygen gas? WebWrite balanced chemical equations for these decomposition reactions. This will give you the number of moles from both the unknown organic molecule and the O2 so you must subtract the moles of oxygen transferred from the O2. WebAluminum Hydrogen Carbonate (aq) B. Let's see how we can determine the chemical formula of this compound. 2) Hydrochloric acid reacts with a solid chunk of aluminum to produce hydrogen gas and aluminum ions. Using the equation #2"Al"(s) + 3"H"_2"SO"_4(aq) -> "Al"_2("SO"_4)_3(aq) + 3"H"_2(g)#. When there is no limiting reagent because the ratio of all the reactants caused them to run out at the same time, it is known as stoichiometric proportions.
Emery is an impure crystalline variety of aluminum oxide. (NIOSH, 2022) Aluminum oxide has a chemical formula Al2O3. It is amphoteric in nature, and is used in various chemical, industrial and commercial applications. It is considered an indirect additive used in food contact substances by the FDA. How many moles #"Ag"_2"O"# are needed to produce #"4.24 mol O"_2"#? In a balanced reaction, both sides of the equation have the same number of elements.
How To Rejoin A Minecraft World After Being Kicked,
Chief Attakullakulla Descendants,
Arhaus Arch Floor Mirror In Brass,
Is It Safe To Spray Lysol In Car Vents,
Articles A
